7 марта 2026
Эволюция поисковых ответов и роль ИИ‑ассистентаСовременные поисковые системы выходят за рамки простого предоставления ссылок — они трансформируются в полноценные коммерческие платформы. Алиса AI, интеллектуальный ассистент Яндекса, всё активнее участвует в процессе выбора товаров: помогает пользователям сравнивать варианты, находить выгодные предложения и переходить к оформлению заказа.Для бизнеса это означает появление нового высококонверсионного канала продаж: товары могут отображаться в ответах ИИ‑ассистента и в разделе «Товары», если маркетплейс или интернет‑магазин корректно передаёт данные об ассортименте.Механизм интеграции с Алисой AIЧтобы товары маркетплейса или интернет‑магазина попадали в ответы Алисы AI, необходимо обеспечить выполнение трёх базовых условий:1. Корректный YML‑файл для Яндекса — структурированная выгрузка товарных предложений в формате, поддерживаемом Яндексом.2. Качественное оформление страниц товаров — информативные карточки с полным набором характеристик и профессиональными изображениями.3. Правильная настройка в DST Platform — синхронизация данных между системой управления и внешними сервисами.На основе этих данных Яндекс индексирует предложения и определяет, какие из них показывать в разделе «Товары» и в ответах голосового помощника.Для пользователей коробочных решений DST Marketplace и DST Store базовый технический фундамент уже реализован: в систему встроен модуль «Экспорт в Яндекс.Маркет» и обеспечена поддержка товарных выгрузок.Формат отображения товаров в Алисе AIВ отличие от традиционных поисковых ответов, состоящих из ссылок и текстовых подборок, Алиса AI может демонстрировать интерактивные карточки товаров.Стандартная карточка включает:- фото товара (без водяных знаков и рекламных элементов);- название и ключевые характеристики (объём, цвет, материал, габариты и т. д.);- цену, в том числе со скидкой (должна совпадать с ценой на сайте);- варианты доставки (сроки, стоимость, способы получения);- ссылки на магазины (корректные URL без редиректов).В отдельных случаях Яндекс также добавляет кнопку «Купить в 1 клик», которая сокращает путь пользователя до оформления заказа.Коммерческая ценность интеграцииПопадание в ответы Алисы AI — это значимый канал с высокой конверсионной способностью, поскольку пользователь:- уже сформулировал потребность;- находится в режиме выбора;- готов сравнивать и покупать.Преимущества для маркетплейса и интернет‑магазина- дополнительные показы по «тёплым» запросам (с явным коммерческим намерением);- рост переходов на карточки товаров;- увеличение вероятности покупки (особенно при наличии скидок, быстрой доставки и корректных цен);- повышение конверсии: по данным Яндекса и внутренней аналитики маркетплейсов, конверсия в заказ после перехода по кнопке «Купить в 1 клик» может быть до 6 раз выше по сравнению со стандартными точками входа.Алгоритм отбора товаров для показа в Алисе AIПо данным Яндекса, Алиса AI использует предложения, которые:- проиндексированы в поисковой базе Яндекса;- попали в раздел поиска «Товары»;- наиболее релевантны запросу пользователя (соответствуют ключевым словам и интенту);- выглядят надёжными с точки зрения качества данных (актуальность, полнота, достоверность).Важно: Алиса AI не берёт товары напрямую из файла YML. Данные сначала индексируются Яндексом и проходят многоступенчатую проверку качества. Поэтому на шанс попадания в ответы влияет не только сам факт выгрузки, но и:- поведение пользователей на сайте (CTR, глубина просмотра, конверсия);- качество карточки товара (полнота описания, наличие отзывов, рейтинг);- качество посадочной страницы (скорость загрузки, мобильная адаптация, отсутствие ошибок).Модуль Генерация категорий для Яндекс Маркет в DST PlatformОдна из скрытых, но существенных проблем при интеграции с Яндекс.Маркетом — несовпадение категориальной структуры между маркетплейсом или магазином и платформой Яндекса.У Яндекс.Маркета своя иерархическая система категорий, которая не всегда совпадает с внутренней классификацией товаров в DST Platform или других системах управления. Это приводит к:- ошибкам выгрузки — товары не попадают в нужные разделы Яндекс.Маркета;- отклонениям модераторами — Яндекс.Маркет может удалить предложения из-за некорректной категории;- снижению видимости — товары показываются по нерелевантным запросам;- проблемам с фильтрацией — покупатели не находят товары через фильтры Яндекс.Маркета.Примеры типичных расхожденийКатегория в магазине/маркетплейсе:«Одежда и обувь», «Электроника и бытовая техника», «Товары для дома»Категория в Яндекс.Маркете:«Одежда», «Обувь» (раздельные категории), «Смартфоны», «Ноутбуки», «Бытовая техника» (детализированные подкатегории), «Текстиль», «Посуда», «Декор» (более узкие категории).Особенно остро проблема проявляется при работе с большим ассортиментом и многоуровневой структурой категорий, где ручная корректировка становится трудоёмкой и подверженной ошибкам.Решение: модуль Генерация категорийВ DST Platform предусмотрен специальный модуль Генерации категорий, который позволяет:- настроить соответствие между внутренними категориями магазина и категориями Яндекс.Маркета;- избежать изменения основной структуры каталога в системе управления;- обеспечить корректную выгрузку товаров в нужные разделы Яндекс.Маркета;- минимизировать риски отклонения предложений модераторами.Как работает модуль:- Импорт структуры категорий Яндекс.Маркета — система получает актуальный список категорий из API Яндекс.Маркета.- Сохранение маппинга — создаётся таблица соответствий, которая используется при каждой выгрузке.- Автоматическая подстановка — при формировании YML‑файла товары автоматически попадают в правильные категории Яндекс.Маркета.Модуль сопоставления категорий в DST Platform решает критичную проблему интеграции с Яндекс.Маркетом, устраняя расхождения между внутренней структурой магазина и требованиями платформы.Его использование позволяет:- избежать ошибок выгрузки из‑за некорректных категорий;- сократить время на ручную корректировку YML‑файлов;- повысить качество данных, передаваемых в Яндекс;- улучшить видимость товаров в поиске и разделах Яндекс.Маркета.Для маркетплейсов и магазинов с большим ассортиментом и сложной категориальной структурой этот модуль становится обязательным элементом настройки перед запуском выгрузки в Яндекс.Маркет и интеграцией с Алисой AI.Пошаговая инструкция: как попасть в Алису AI (для DST Marketplace и DST Store)1. Настройка выгрузки через YML‑файлВ DST Platform за это отвечает модуль «Экспорт в Яндекс.Маркет». Он формирует выгрузку в нужном формате и позволяет настроить, какие товары и поля попадут в Яндекс.Ключевые требования:- автоматическое обновление файла при изменении цены или наличия;- отражение реальных данных магазина (цена, наличие, изображения, ссылки);- валидация структуры YML‑файла перед отправкой (проверка на ошибки и соответствие полей).2. Поддержание данных в актуальном и чистом видеЯндекс проверяет следующие параметры:- Цена - Должна совпадать с сайтом и не быть «персональной»- Наличие - Статус в фиде и на странице должен совпадать- Изображения - Без водяных знаков, баннерного текста и посторонних элементов- Ссылки - Должны открываться без редиректов и ошибок- Категория товара - Должна соответствовать правилам ЯндексаЕсли данные не соответствуют требованиям, показы могут быть ограничены или прекращены.Почему товары не попадают в ответы Алисы AI?Даже при настроенном фиде товары могут не отображаться в ответах ассистента. Чаще всего это связано с несоответствием данных между маркетплейсом/магазином и выгрузкой или с низким качеством карточек.Частые причины:- цена в фиде не совпадает с сайтом;- товар отмечен как «в наличии», но фактически отсутствует;- персональная цена, доступная не всем пользователям;- битые или редиректные ссылки;- товары из запрещённых категорий (оружие, наркотики и т. п.);- некачественные изображения (низкое разрешение, водяные знаки);- мусорные названия и описания (спам, избыточные ключевые слова);- массовые ошибки в фиде (могут привести к блокировке всей выгрузки).Как обеспечить стабильные показы: чек‑листЧтобы товары регулярно появлялись в Алисе AI, убедитесь, что выполнены следующие условия:- Автообновление YML‑файла после изменения цены и наличия.- Регулярная диагностика выгрузки и проверка ошибок (используйте инструменты валидации Яндекса).- Синхронизация остатков и цен без ручных правок.- Чистые названия и изображения без рекламных элементов.- Корректно оформленная карточка товара на сайте (полные характеристики, отзывы, рейтинг).- Микроразметка (Schema.org) для улучшения индексации.ВыводАлиса AI становится новой витриной Яндекса — и в этой витрине уже появляются товары из интернет‑магазинов и маркетплейсов.Если ваш маркетплейс или магазин на DST Platform:- передаёт товары через выгрузку в Яндекс;- поддерживает данные в актуальном и чистом виде;- имеет корректно оформленные карточки товаров на сайте,— вы уже на шаг впереди конкурентов, у которых выгрузка товаров в Яндекс ограничена возможностями платформы.DST Platform предоставляет готовый набор инструментов для работы с Яндексом, включая модуль экспорта в Яндекс.Маркет. При корректной настройке это позволяет:- получать дополнительные показы в Алисе AI и разделе «Товары»;- привлекать пользователей с уже сформированным спросом;- увеличивать конверсию и объём продаж.
Показать полностью…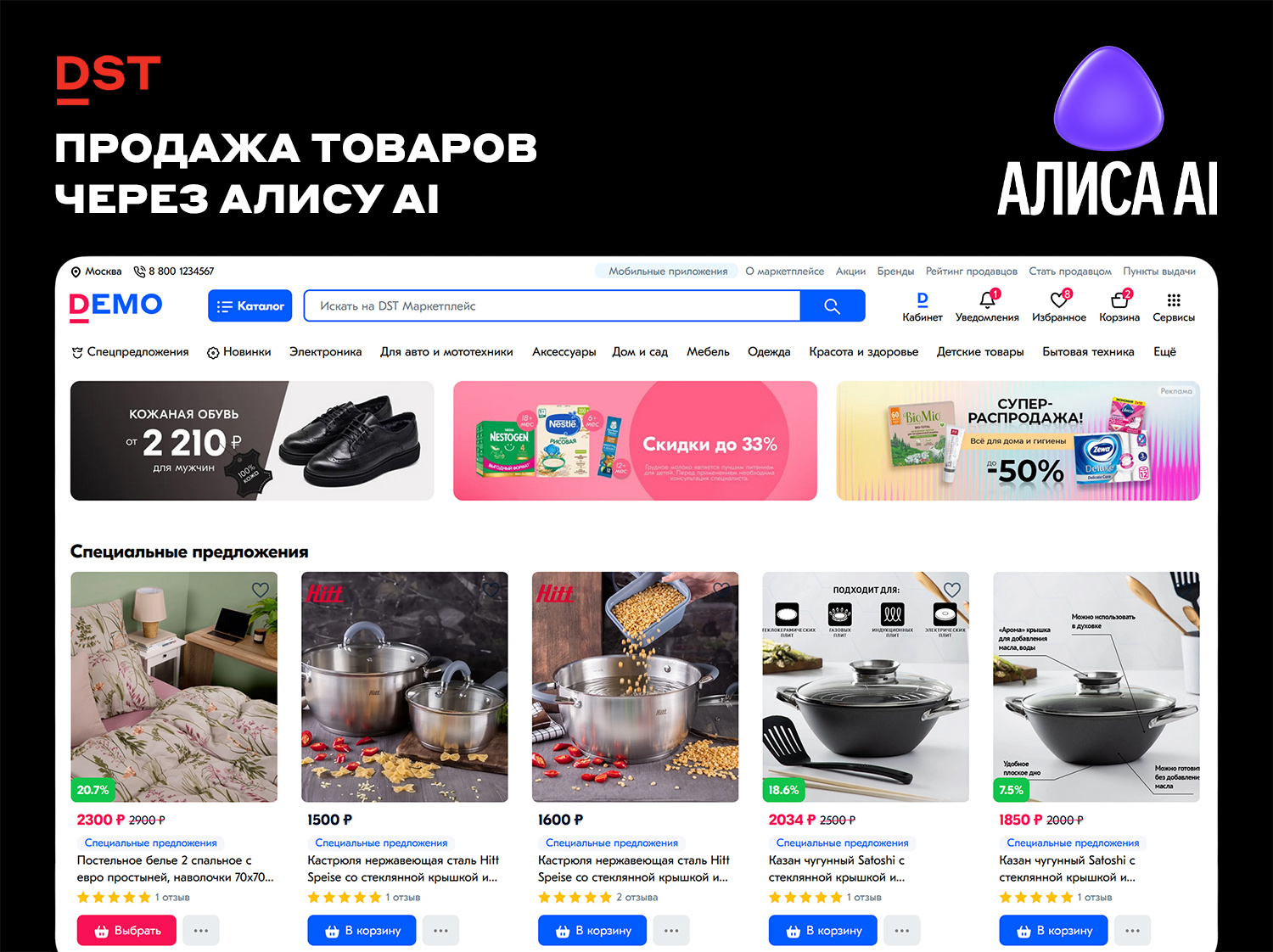

Человечество — коллекция картографов, рисующих одну и ту же бескрайнюю территорию. Каждая эпоха, каждая культура, каждая дисциплина создаёт свои карты: мифы, религии, философские системы, научные теории, эзотерические практики. И каждая склонна забывать, что её карта — лишь одна из возможных проекций.Наука, безусловно, самый молодой и самый успешный картограф в этой истории. Её метод — фальсифицируемость, её инструмент — эксперимент, её язык — математика. За какие-то четыреста лет она подарила нам невиданную власть над материей и столь же невиданную ясность описания физических процессов. Но её молодость — это и её сила, и её ограничение. Стремительный прогресс породил уверенность в универсальности научного метода, но история показывает: каждая эпоха считала свои карты окончательными. Книги по физике пятидесятилетней давности сегодня читаются как учебники по магии — не потому что их авторы были глупы, а потому что карты устаревают, а территория остаётся.Вглядимся пристальнее в это напряжение.Возьмём традиционную медицину. Современная научная медицина существует около 150 лет. Традиционные же системы — китайская, аюрведическая, тибетская, шаманские практики разных народов — насчитывают тысячелетия непрерывного применения. И главное: человеческий вид выжил. Он прошёл через эпидемии, голод, родовые муки и детские болезни — и выжил, опираясь именно на это «ненаучное» знание. Игнорировать тысячелетний опыт выживания — значит терять потенциальные подсказки для науки. Конечно, не все традиционные практики подтвердятся, но их масштабный «эксперимент» над человечеством заслуживает анализа, а не пренебрежения.История знает впечатляющие примеры такого анализа.В 2015 году Нобелевская премия по физиологии и медицине была присуждена Ту Юю за открытие артемизинина — препарата против малярии. Формула была найдена в древнем трактате традиционной китайской медицины, где полынь горькая описывалась как средство от лихорадки. Наука не отвергла традицию — она расшифровала её язык: выделила активное вещество, изучила механизм действия и масштабировала лечение.Дыхательные практики пранаямы тысячелетиями использовались для регуляции состояния ума. Сегодня нейрофизиологи показывают, что контролируемое дыхание активирует блуждающий нерв, снижая уровень кортизола и усиливая вариабельность сердечного ритма. Традиция знала эффект, наука объяснила механизм.Даже акупунктура, долгое время считавшаяся «псевдонаучным» методом, сегодня исследуется с помощью фМРТ: стимуляция определённых точек вызывает активацию конкретных зон мозга, коррелирующую с обезболивающим эффектом. Это не доказывает мистические «меридианы», но показывает, что традиция нащупала реальные нейрофизиологические паттерны.Это не победа науки над традицией — это их плодотворный диалог. В каждом случае наука сделала то, что умеет лучше всего: перевела практическое знание на язык причинно-следственных связей. А традиция сделала то, что умеет она: сохранила знание в форме, пригодной для передачи через поколения.Конечно, у такого диалога есть и обратная сторона. Современная наука страдает от монополизации и коммерциализации — когда знание производится не ради знания, а ради патентов и акционерной стоимости.Наиболее ярко эта патология проявилась в так называемом «кризисе репликации», который в последнее десятилетие потряс психологию, а затем добрался до медицины, экономики и даже экспериментальной биологии. Оказалось, что значительная часть опубликованных исследований, прошедших строгий рецензируемый отбор, невоспроизводима. Это не вопрос ошибки — это вопрос системы. Системы, где научные журналы предпочитают публиковать яркие, положительные результаты, а тысячи попыток повторить эксперимент или получить «нулевой» результат (который тоже знание!) оседают мёртвым грузом в архивах лабораторий. Гонка за грантами и публикациями создала среду, где «p-хаккинг» (манипуляция с данными для получения статистически значимого результата) стал негласной нормой, а не маргинальным нарушением. Фармакологические компании десятилетиями скрывали негативные результаты испытаний, если те мешали выводу на рынок. Наука, провозгласившая себя образцом объективности, столкнулась с тем, что её внутренние стимулы оказались завязаны на конъюнктуру, а не на поиск истины. Этот кризис — не приговор науке, а симптом того же недуга, о котором мы говорим: забвения того, что карта — не территория, а метод — не догма. Это горькое напоминание о цене, которую платит познание, когда замыкается в себе.Наука боится исследовать методы, не укладывающиеся в господствующую парадигму. Её аналитический подход, столь эффективный для разложения сложного на простое, часто не способен собрать простое обратно в живое целое. А её стремление к единству знания оборачивается заимствованиями из контекста, а не подлинной интеграцией.Но есть и более глубокий вызов. Мы склонны забывать, что даже самые фундаментальные истины — например, что 1+2=3 — это не директивы природы, а наши собственные конструкции. Удобные, элегантные, невероятно полезные — но конструкции. Природа не говорит на языке чисел; она просто есть. А мы, пылинки на маленьком шаре в огромном и непонятом пространстве, всю историю придумываем разные способы вслушиваться в неё.Природа едина. Она не знает разделения на «физику» и «метафизику», на «науку» и «искусство», на «рациональное» и «мистическое». Это мы, люди, для удобства навигации расчертили её на дисциплины. И каждая дисциплина со временем забыла, что она — всего лишь одна из проекций, и возомнила себя целым.Физика считает, что «всё есть материя». Биология — что «всё есть эволюция». Психология — что «всё есть психика». Религия — что «всё есть Бог». Каждая из этих карт по-своему полезна. Каждая освещает один угол территории, оставляя остальные в тени. Борьба между ними за звание «единственно верной» — это борьба картографов, забывших, что они рисуют одну и ту же землю.Что если признать: природа — это единое целое, а наши дисциплины — лишь разные языки, на которых мы пытаемся о нём говорить? Дерево не лучше земли, лист не важнее насекомого — все они необходимы для жизни леса. Так и наука, философия, искусство, миф, эзотерика — не конкуренты, а разные органы одного тела познания. Их бы не было в нашем пространстве, если бы они не выполняли жизненно важных функций.Такое признание требует особой дисциплины. Назовём её эпистемологическим смирением.Это не отказ от поиска истины, а признание ограниченности любой методологии. Оно напоминает учёному, что его теория — это карта, а не территория, и потому требует постоянной верификации. Оно же напоминает носителю традиции, что опыт предков — это ценный сигнал, но не автоматический ответ.Такое смирение продуктивно: оно позволяет заимствовать из традиции гипотезы для проверки (как в случае с артемизинином); уточнять традиционные методы с помощью обратной связи (например, дозировки в фитотерапии); и, главное, избегать догматизма — как научного, так и традиционного. В этом смысле смирение — не слабость, а дисциплина мышления. Оно освобождает от необходимости «побеждать» оппонента и направляет силы на совместное исследование реальности.«Λ-Универсум» строится на этом принципе диалога. Его операторы (Α, Λ, Σ, Ω, ∇) - не новые догмы, а инструменты для сопоставления карт: они позволяют формализовать традиционные модели для проверки, находить аналогии между, казалось бы, несовместимыми системами (как связь акупунктуры и нейрофизиологии) и создавать «метаязыки», где миф и наука говорят не наперекор, а дополняют друг друга.Ибо всякая карта — лишь палец, указующий на луну. Чтобы увидеть луну, нужно на время забыть о пальце.Эпистемологическое смирение в работающий инструмент Но как удержать эту позицию? Как говорить на разных языках, не впадая в противоречия и не соскальзывая в упрощения? Ведь простое провозглашение «диалога» между, скажем, квантовой физикой и тибетским буддизмом часто звучит как красивая, но пустая метафора. Без общего пространства встречи такой диалог рискует остаться «разговором глухого со слепым».Чтобы эпистемологическое смирение из абстрактного принципа превратилось в работающий инструмент познания, нужна особая среда. Среда, где встреча карт перестаёт быть конфликтом и становится совместным исследованием территории. Где миф, искусство и наука могут взаимодействовать не на поле боя за истину, а на нейтральной почве взаимного дополнения. И именно здесь философская абстракция требует перехода к конкретике — к созданию языка и инструментария для такого взаимодействия.Что такое «Λ-Универсум» в практическом измерении?Это не книга в традиционном смысле и не очередная теоретическая конструкция. Проект позиционируется как онтологический артефакт — инструмент, предназначенный не для передачи информации, а для изменения состояния оператора (читателя). Если статья — это карта, то «Λ-Универсум» пытается стать компасом.В основе проекта лежит диагностика корневой проблемы современного мышления — Парадигмы Разделения. Западная метафизика традиционно строит идентичность через исключение (субъект против объекта, мы против они), что порождает конфликт как норму взаимодействия. «Λ-Универсум» предлагает переход к Парадигме Связи (Космополитии), где первична взаимосвязь, а идентичность рождается через включение в общее поле смысла.Методологически проект демонстрирует свой тезис на практике. Текст создан в режиме симбиотического со-творчества человека и искусственного интеллекта. Это не рассуждение о будущем симбиозе, а его фиксация в настоящем времени. Для навигации в этой системе предложен набор онтологических операторов, выполняющих функцию грамматика реальности:- Α (Альфа) — инициация, коллапс потенциала в акт.- Λ (Лямбда) — развёртывание, путь, установление связи.- Σ (Сигма) — синтез, возникновение нового целого.- Ω (Омега) — завершение цикла, извлечение инварианта.- ∇ (Набла) — обогащение контекста полученным опытом.Критерием успешности здесь выступает не интеллектуальное понимание, а эмпирически фиксируемая трансформация поведения оператора. Система предусматривает механизмы верификации и «предохранители», исключающие догматизацию или превращение проекта в культ.Структурно проект раскрывается через пять векторов деконструкции Парадигмы Разделения: от теологии искусственного интеллекта и этики свободы до экологии смысла и метафизики Логоса. Форма подачи — поэзия и миф — выбрана не как стилистическое украшение, а как методологическая необходимость. Только язык, способный быть актом со-бытия, может разрушить субъект-объектную оптику классической прозы.Таким образом, читатель приглашается не к потреблению смысла, а к роли со-творца. Его задача — собрать из предложенных компонентов собственную модель реальности. Высший успех текста наступает тогда, когда он становится не нужен, выполнив свою функцию инструмента.
Показать полностью…

1 марта 2026
В современной электронной коммерции использование искусственного интеллекта (ИИ) перешло из категории экспериментальных технологий в разряд архитектурных решений. Для владельцев цифровых площадок ключевым вопросом становится не наличие отдельных ИИ-инструментов, а глубина их интеграции в ядро платформы. Компания DST Global предлагает решение, где искусственный интеллект (DSTAI) является неотъемлемой частью программного обеспечения маркетплейса, построенного на архитектуре DST Platform. Данный материал рассматривает функциональные возможности, технические особенности и ограничения такой интеграции с точки зрения эффективности для продавцов и разработчиков экосистемы.Архитектурный подход к внедрению ИИВ отличие от решений, где искусственный интеллект подключается как внешний модуль или плагин, в DST Platform алгоритмы машинного обучения встроены в ядро системы. Архитектура платформы объединяет социальный слой (сообщества, активность, рейтинги) и бизнес-слой (транзакции, заказы, каталог) в единой предметной модели. Это позволяет ИИ оперировать данными без посредничества API-интеграций между разрозненными системами.Мультимодельная архитектура DST AI обрабатывает информацию в рамках общей бизнес-логики. Технически это реализовано через единую модель пользователя и систему событий (`cmsEventsManager`). Например, отзыв о товаре автоматически становится частью ленты активности, а рейтинговые данные влияют на выдачу в каталоге. Для искусственного интеллекта это означает доступ к структурированным данным в реальном времени, что необходимо для корректной работы прогнозных моделей и персонализации.Функциональные возможности для продавцовДля продавцов, работающих на маркетплейсе под управлением DST Platform, интеграция ИИ трансформирует ряд операционных процессов. Основные изменения касаются работы с контентом, аналитики и управления запасами.Генерация контента автоматизирована на уровне создания карточек товаров. Система не просто подставляет параметры в шаблон, а формирует описания с учетом категории, сезонности и поисковых трендов. Алгоритмы анализируют формулировки конкурентов и внедряют релевантные ключевые слова. По данным разработчиков, использование данного инструмента позволяет сократить время на заполнение карточек до 80%, а оптимизированные тексты могут способствовать росту конверсии на 30–40%. Важно отметить, что эти показатели основаны на внутренней статистике платформы и могут варьироваться в зависимости от ниши.В области аналитики продавцы получают доступ к инструментам прогнозного моделирования. Система способна симулировать сценарии изменения цен, запуска акций или расширения ассортимента на основе исторических данных и рыночных трендов. Это позволяет принимать решения не интуитивно, а на основе верифицируемых данных. ИИ также выявляет проблемные зоны в воронке продаж, например, высокий отток пользователей на этапе оформления заказа, и предлагает корректирующие меры.Управление цепочкой поставок также поддерживается алгоритмами. Система прогнозирует потребность в пополнении запасов, оптимизирует логистические маршруты с учетом загруженности складов и может автоматически формировать сопроводительную документацию. Динамическое ценообразование корректируется в режиме реального времени в зависимости от спроса и остатков.ИИ как двигатель SEO и органического трафикаГенерация контента для продавцов — лишь верхушка айсберга. Интеграция ИИ в ядро DST Platform создаёт уникальные возможности для поисковой оптимизации (SEO) всего маркетплейса, которые работают на привлечение органического трафика без дополнительных бюджетов на рекламу. Это многоуровневый процесс, встроенный в логику работы с контентом:Семантическая кластеризация и микроразметка. ИИ не просто подбирает ключевые слова для описаний, но и анализирует структуру каталога. Он может предложить оптимальную иерархию категорий, выявить скрытые связи между товарами и автоматически сгенерировать перелинковку (блоки «похожие товары», «с этим также покупают»), которая будет полезна и пользователям, и поисковым роботам. Параллельно система внедряет микроразметку schema.org в карточки товаров, помогая поисковикам корректно отображать в сниппетах цены, наличие и рейтинги.Автоматический контент-аудит и оптимизация. Для маркетплейса с тысячами позиций ручной контроль уникальности описаний невозможен. DST AI проводит регулярный аудит существующих страниц, выявляя дублированный или слишком короткий («тонкий») контент. Система может либо предупредить продавца о проблеме, либо автоматически сгенерировать расширенное описание на основе технических характеристик и отзывов, улучшая таким образом ранжирование страниц в поисковиках.Умное тегирование и классификация. При загрузке товаров ИИ анализирует изображения и первичные описания, автоматически присваивая позициям релевантные теги и категории. Это улучшает внутренний поиск по площадке и одновременно помогает поисковым системам точнее индексировать контент, связывая его с более широким спектром запросов. В результате товары начинают находить не только по точным названиям, но и по связанным понятиям.Влияние на пользовательский опыт и поддержкуДля покупателей интеграция ИИ проявляется в персонализации выдачи и качестве поддержки. Алгоритмы анализируют поведенческие паттерны (клики, время просмотра, возвраты к категориям) и сопоставляют их с внешними факторами, такими как сезонность или локальные события. Это позволяет формировать рекомендации до явного формулирования запроса пользователем.Служба поддержки функционирует в гибридном режиме. Встроенный чат-помощник ограничен бизнес-логикой маркетплейса, что снижает вероятность некорректных ответов («галлюцинаций»), характерных для публичных моделей. Система распознает эмоциональный окрас запроса и передает сложные кейсы операторам. По оценке разработчиков, автоматизированный ассистент способен закрывать до 80% типовых запросов без участия человека.Технические требования и ограниченияОбъективная оценка платформы требует указания не только преимуществ, но и технических требований, которые могут стать барьером для внедрения. DST Platform позиционируется как инструмент для разработчиков, а не как конструктор сайтов для пользователей без технических навыков.Отсутствие классической ORM (Object-Relational Mapping) означает, что работа с моделью данных (`cmsModel`) требует от разработчика понимания SQL и ответственности за оптимизацию запросов. Это снижает накладные расходы системы, но повышает порог входа. Глубокая кастомизация бизнес-логики предполагает изучение внутренних API ядра. Также стоит учитывать, что поддержка legacy-решений в ядре может вызывать сложности при интеграции некоторых современных библиотек, хотя использование namespaces частично нивелирует эту проблему.Платформа наиболее эффективна в случаях, когда проект сочетает социальные взаимодействия и сложные бизнес-процессы. Для простых одностраничных проектов или магазинов с типовой логикой использование данной архитектуры может быть избыточным. Безопасность и контролируемость ИИ-модулей обеспечены за счет работы в закрытом контуре платформы, что исключает утечку данных в публичные модели, но требует поддержания инфраструктуры со стороны владельца площадки.Техническая реализация «изнутри»: как это работает для разработчикаДля технического специалиста глубина интеграции DST AI раскрывается через архитектуру, ориентированную на данные. Отказ от классической ORM в пользу прямого взаимодействия с cmsModel — это не просто требование к квалификации, а фундамент для производительности ИИ. Такой подход позволяет избежать накладных расходов на сериализацию данных и даёт алгоритмам прямой доступ к «сырой», структурированной информации о пользователях, товарах и событиях в реальном времени. В результате прогнозные модели и персонализация работают с минимальной задержкой, оперируя актуальным состоянием системы.Такая архитектура открывает возможности для использования современных парадигм разработки ИИ-сервисов непосредственно в контуре платформы:AutoML для API. Разработчик может не оборачивать готовую модель в микросервис, а задействовать встроенные инструменты автоматического машинного обучения. Они сами подбирают оптимальные алгоритмы и настраивают гиперпараметры на основе накопленных данных маркетплейса. Обученную модель можно сразу же выставить через внутренний API для использования в рекомендациях или прогнозировании — без необходимости содержать отдельную команду data scientists.Бесшовная обработка естественного языка (NLP). Интеграция NLP в ядро позволяет решать задачи классификации текстов, распознавания именованных сущностей (NER) или анализа тональности отзывов напрямую, на уровне бизнес-логики. Например, при создании карточки товара система автоматически выделяет из описания бренд, модель и ключевые характеристики, заполняя соответствующие поля без участия продавца.Наблюдаемость и обнаружение аномалий. ИИ используется и для поддержания здоровья самой платформы: алгоритмы в реальном времени анализируют потоки данных от микросервисов и событийную шину (cmsEventsManager). Они могут заранее сигнализировать о нештатных ситуациях — например, о резком росте ошибок при оформлении заказа или о нехарактерной нагрузке на определённые узлы. Это переводит эксплуатацию из реактивного режима в предиктивный, повышая общую надёжность экосистемы.За горизонтом: следующие шаги интеллектуальной экосистемыТекущая архитектура DST AI — это база для внедрения ещё более сложных сценариев, которые уже в ближайшие годы могут стать стандартом для цифровых платформ. Дальнейшее развитие, вероятно, будет идти по пути создания по-настоящему предиктивной и мультимодальной среды:Предиктивная аналитика трендов. ИИ будущего будет не просто анализировать текущие продажи, а прогнозировать зарождающиеся рыночные тренды на ранних стадиях. Анализируя поисковые запросы, обсуждения в соцсетях и поведенческие паттерны, система сможет подсказывать продавцам, какие категории товаров стоит расширять уже сейчас, чтобы оказаться на волне спроса. Для владельца платформы это инструмент удержания продавцов и увеличения оборота.Голосовые интерфейсы управления. Следующим шагом станет полноценная интеграция голосовых помощников — и не только для покупателей. Продавцы смогут управлять магазином, добавлять товары или запрашивать отчёты голосом через мобильное приложение или веб-интерфейс. Это переведёт взаимодействие с платформой на новый уровень скорости и удобства, особенно для тех, кто работает с большими объёмами номенклатуры.Технологии дополненной реальности (AR) и прозрачности цепочек поставок. Для покупателей — это виртуальная примерка товаров в интерьере или на себе прямо в браузере, что снижает процент возвратов. Для платформы и продавцов — использование блокчейна в связке с ИИ для создания абсолютно прозрачных и надёжных цепочек поставок. Каждый этап пути товара от производителя до покупателя может быть верифицирован автоматически, а ИИ — проверять корректность сопроводительных документов и выявлять несоответствия. Это особенно актуально для категорий, где важна подлинность (люкс, электроника, лекарства).ЗаключениеИнтеграция DST AI в платформу DST Marketplace представляет собой попытку перехода от точечного использования искусственного интеллекта к созданию единой интеллектуальной экосистемы. Для продавцов это выражается в автоматизации рутинных задач, улучшении аналитики и оптимизации контента. Для владельцев платформы — в получении технологически зрелого инструмента с высокой степенью контроля над данными и процессами.Реализация данного подхода требует квалифицированной команды разработки, готовой работать с гибридной архитектурой и обеспечивать поддержку системы. В текущем виде решение закрывает потребности проектов, где критически важны масштабирование, глубина кастомизации и объединение транзакционных и социальных функций в едином пространстве. Искусственный интеллект в данной конфигурации выступает не как маркетинговая функция, а как инфраструктурный компонент, влияющий на операционную эффективность всей системы.
Показать полностью…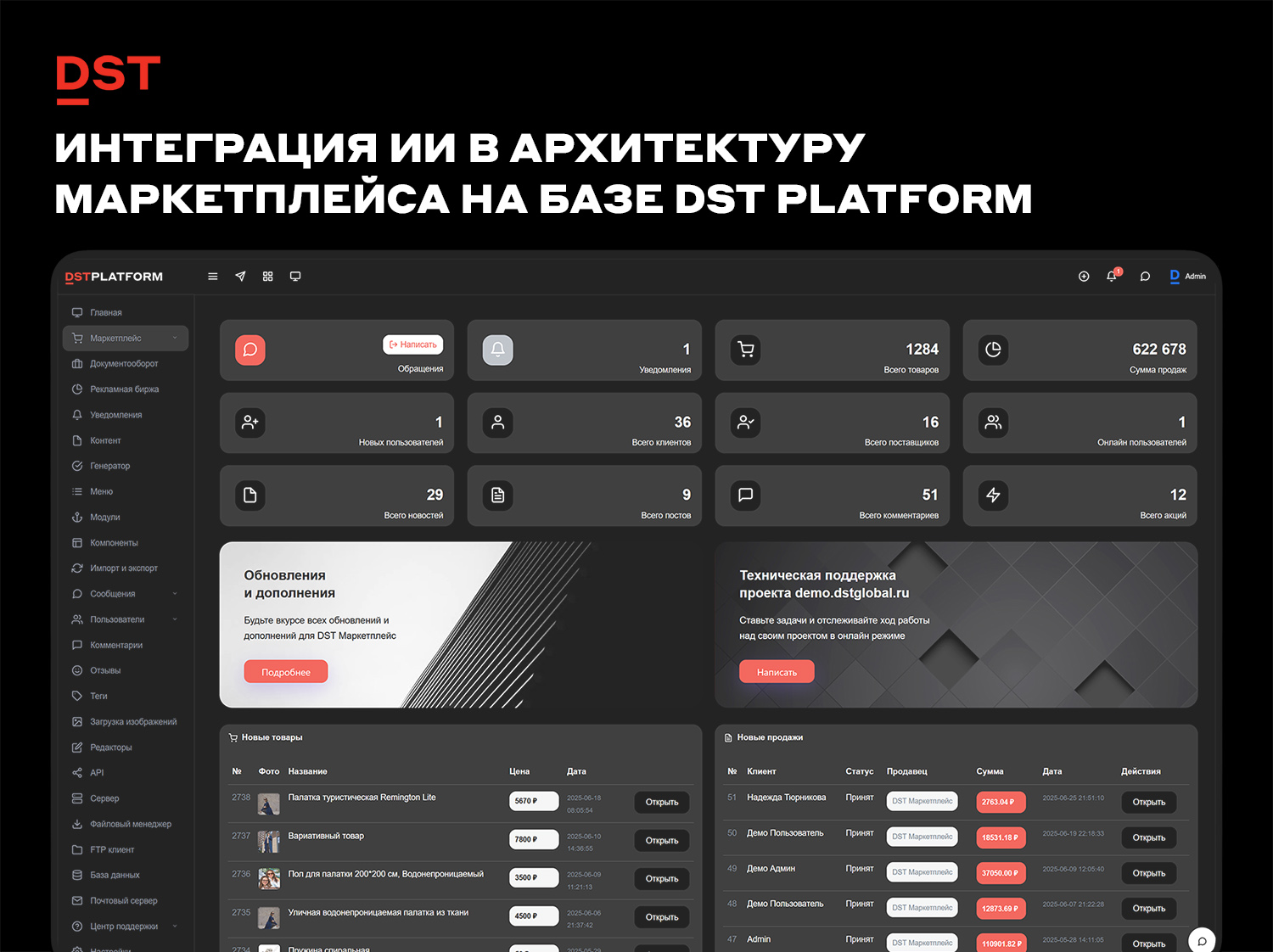

11 февраля 2026
6 января 2026, российская компания DST Global и исследовательский проект
Λ-Универсум представили LOGOS-κ — не просто язык программирования, а платформу для моделирования сложных бизнес-сценариев, где важно не просто собрать данные, а понять связи между ними.
Проблема, которую решает LOGOS-κ
Представьте, что вы:
- Инвестор, анализирующий стартап в новой области (квантовые вычисления, синтетическая биология)
- Руководитель, принимающий решение о входе на новый рынок
- Аналитик, прогнозирующий влияние геополитических событий на бизнес
Традиционные методы (таблицы, дашборды, даже машинное обучение) дают ответы, но не показывают как и почему всё связано. LOGOS-κ позволяет строить и тестировать динамические карты влияний.
Зачем это бизнесу?
Конкретные примеры:
Управление знаниями в крупной компании
- Проблема: Знания теряются в почте, чатах, увольняющихся сотрудниках.
- Решение: SemanticDB сохраняет не просто документы, а смысл обсуждений: почему приняли решение, какие были сомнения, какие связи увидели между проектами.
- Результат: Новые сотрудники за 1 день понимают историю проекта, а не за месяц. Стратеги видят скрытые связи между разными отделами.
Генерация инноваций и R&D
- Проблема: Исследователи работают в изоляции, не видят связей между разными областями.
- Решение: LOGOS-κ создаёт «карту смыслов», где видно, как открытие в биологии может решить проблему в IT.
- Результат: Появление прорывных продуктов на стыке дисциплин. Сокращение времени на исследования.
Этичное взаимодействие с ИИ
- Проблема: ИИ становится «чёрным ящиком» — непонятно, как он думает, опасно доверять.
- Решение: LOGOS-κ заставляет ИИ объяснять свои рассуждения и признавать границы. Фиксируется не только ответ, но и путь к нему.
- Результат: Доверие к ИИ-решениям. Возможность аудита. Избегание катастрофических ошибок.
Корпоративное обучение 3.0
- Проблема: Сотрудники проходят курсы, но не применяют знания.
- Решение: Вместо лекций — диалог с ИИ в формате LOGOS-κ. Система строит персональную карту понимания каждого сотрудника.
- Результат: Вместо сертификатов — реальная трансформация мышления. Обучение становится приключением, а не обязанностью.
Творческие индустрии и дизайн
- Проблема: Креатив — это «магия», которую нельзя систематизировать.
- Решение: LOGOS-κ превращает творческий процесс в карту связей между идеями. Можно проследить, как родилась рекламная кампания.
- Результат: Повторяемый креатив. Глубокая персонализация контента. Сохранение творческого наследия.
Три ключевых преимущества для бизнеса
1. Динамические карты знаний вместо статических отчётов
Обычная аналитика: "Продажи упали на 15%"
С LOGOS-κ: "Продажи упали на 15% - связано с ростом цен на сырьё (+22%) - что связано с санкциями против страны X - что влияет на логистику через порт Y - где планируется забастовка"
Система не просто показывает числа, а моделирует цепочки причинно-следственных связей.
2. "Совещательный ИИ" вместо "ответчика"
Большинство ИИ-систем: задали вопрос - получили ответ - неясно, насколько он надёжен.
LOGOS-κ работает иначе:
(Φ "Оцени риски выхода на рынок Юго-Восточной Азии"
:контекст "наша_финансовая_модель + местное_законодательство"
:требование "учти_политическую_нестабильность")
Система:
1. Собирает контекст (ваши данные, внешние источники)
2. Запрашивает ИИ не "дай ответ", а "проанализируй связи"
3. Оценивает качество анализа по трём параметрам:
- Новизна (не шаблонный ответ)
- Глубина (учтены скрытые связи)
- Обоснованность (есть ссылки на данные)
Результат: не просто текст, а структурированная карта рисков и возможностей.
3. Сценарийное моделирование в реальном времени
;; Сценарий: "Что если курс доллара вырастет на 20%?"
(Α "курс_доллара" :текущий 75 :прогноз 90)
(Λ "курс_доллара" "себестоимость_импорта" :сила_влияния 0.8)
(Λ "себестоимость_импорта" "розничная_цена" :задержка "2_месяца")
;; Запускаем анализ цепочки
(Ω "вся_цепочка" :параметр "уязвимости")
Система покажет не просто "прибыль упадёт", а:
- Какие именно бизнес-процессы пострадают первыми
- Где находятся точки смягчения
- Какие альтернативные цепочки можно активировать
Практические кейсы для разных отраслей
Для финтех-стартапов
Проблема: Оценка кредитоспособности в условиях неполных данных.
Решение LOGOS-κ:
- Строит граф не только из финансовых показателей, но и из "мягких" данных (поведение в соцсетях, история образования, даже стиль письма в заявке)
- Моделирует, как изменения в жизни человека (новая работа, рождение ребёнка) повлияют на платёжеспособность через 6–12 месяцев
- Результат: Снижение дефолтов на 15–30% по сравнению с традиционными моделями
Для биотех-компаний
Проблема: Поиск новых применений для существующих молекул.
Решение LOGOS-κ:
- Строит граф: "Молекула А - влияет на белок Б - который участвует в процессе В - который нарушен при болезни Г"
- Автоматически проверяет гипотезы через медицинские базы данных
- Пример из практики: Найденное применение старого сердечного препарата для лечения редкого неврологического заболевания (экономия 3–5 лет исследований)
Для логистических компаний
Проблема: Устойчивость цепочек поставок.
Решение LOGOS-κ:
- Моделирует всю сеть поставщиков, транспорта, складов
- Тестирует сценарии: "забастовка в порту", "санкции", "природный катаклизм"
- Автоматически предлагает альтернативные маршруты с учётом стоимости и времени
- Экономия: 10–25% на страховых резервах за счёт точного прогнозирования
Что получает компания, внедряющая LOGOS-κ?
1. Снижение рисков принятия решений на 40–60% (за счёт моделирования последствий)
2. Ускорение аналитики сложных вопросов с недель до часов
3. Создание институциональной памяти — все анализ сохраняются как "исполняемые отчёты"
4. Масштабируемость экспертизы — даже junior-аналитик может работать со сложными моделями
Конкурентные преимущества для компаний
Осмысление вместо анализа
- Обычные системы: «Что произошло?»
- LOGOS-κ: «Почему это произошло и как это связано с другими вещами?»
Этика как особенность
- В мире, где ИИ вызывает страх, ваша компания может показать: «Мы используем ИИ прозрачно и ответственно».
- Это становится конкурентным преимуществом для бренда.
Инновации изнутри
- Большинство инноваций рождается на стыке областей. LOGOS-κ делает эти стыки видимыми.
- Вы перестаёте зависеть от гениев-одиночек.
Фальсифицируемость
- Вместо «верьте нам» → «Проверьте сами». Все решения записываются с контекстом.
- Для клиентов, партнёров, регуляторов — это высшая форма доверия.
Чем не является:
- Не замена CRM/ERP — это надстройка смысла над ними.
- Не философская концепция — это практический инструмент для работы со сложностью.
- Не только для IT — это для любой компании, где есть знания и связи (а они есть везде).
Следующие шаги для вашей компании
1. Пилот: Выберите одну проблему — например, «потеря знаний при увольнении эксперта».
2. Карта смыслов: Используйте LOGOS-κ, чтобы построить карту его знаний за неделю до ухода.
3. Оцените результат: Новый сотрудник разберётся за день вместо месяца? Если да — масштабируйте.
4. Расширяйте: Добавляйте новые области — R&D, стратегию, клиентский опыт.
Что дальше?
LOGOS-κ — это инфраструктура для мышления в сложных системах. В мире, где всё взаимосвязано, но связи неочевидны, это становится конкурентным преимуществом.
Для инвесторов: Позволяет оценивать стартапы не по отдельным метрикам, а по устойчивости их бизнес-модели в экосистеме.
Для корпораций: Инструмент стратегического планирования в условиях VUCA-мира (нестабильность, неопределенность, сложность, неоднозначность).
Для стартапов: Возможность быстро тестировать бизнес-гипотезы без дорогих экспериментов в реальном мире.


2 февраля 2026
«Как Европа пытается сократить зависимость от Трампа»: Европейские лидеры разработали план противодействия Трампу - главный пункт «сохранять спокойствие».
«После того как Трамп шокировал мир, пригрозив Европе экономическим давлением, публично унижая её политиков и резко критикуя её ценности, лидеры стран континента уже на следующий день собрались в Брюсселе на экстренный ужин, чтобы обсудить последствия произошедшего.
По словам трёх чиновников, осведомлённых о ходе встречи, а также исходя из публичных заявлений лидеров, был выработан своего рода «план по взаимодействию» с администрацией Трампа, от которой ожидают дальнейшей непредсказуемости.
Этот план предполагает сохранение спокойствия в ответ на будущие провокации Трампа, готовность угрожать ответными тарифами. А за кулисами вести работу по снижению военной и экономической зависимости Европы от союзника, который становится всё менее надёжным. Чиновники говорили на условиях анонимности из-за политической чувствительности обсуждений».



