berry cristan
9 февраля 2023
CAR-T Cell Therapies: Addressing Key Unmet Needs Across Various Oncological Indications
Amidst the active initiatives undertaken to develop more targeted anti-cancer therapies, CAR-T therapies have emerged as a promising option, given their ability to eradicate tumor cells from the body with minimal treatment-related side effects.
Further, CAR-T cell therapies, relatively recent addition to the gamut of anticancer interventions, has demonstrated significant promise. Overall, this highly specific and promising form of CAR-T cell therapy treatment, which harnesses the versatile effector machinery of the human immune system, has revolutionized cancer treatment, globally. Given the consistent increase in number of cell therapies being developed and launched, this upcoming therapeutic segment is on its way to becoming one of the highest valued markets within the biopharmaceutical industry.
At present, more than 5 CAR-T therapies have been approved for several hematological malignancies, including KYMRIAH® (Novartis), YESCARTA® (Gilead Sciences), TECARTUS™ (Gilead Sciences), Breyanzi® (Bristol Myers Squibb), Abecma™ (Bristol Myers Squibb) and CARVYKTI™ (Janssen Biotech / Legend Biotech). In fact, more than 170 companies are engaged in the development of over 970 early and late-stage CAR-T therapies, worldwide. Moreover, several promising leads are anticipated to be commercially launched over the coming decade, following which the market is projected to grow at a substantial pace.
Over 6,500 patents related to CAR-T cell therapies have been recently filed / granted, demonstrating the continued innovation in this domain. In addition, more than 260 collaborations have been inked between several industry / academic stakeholders in order to advance the development of various pipeline candidates. To fund product development initiatives, capital investments worth more than USD 24 billion have been made by various private and public sector investors, in the last few years. Driven by the ongoing pace of innovation in this field, sufficient financial support from investors and encouraging clinical trial results, the CAR-T cell therapies market is likely to witness significant growth in the foreseen future.
CAR-T Cell Therapies, With Over 970 Preclinical / Clinical Candidates, Represent One of The Most Active Segments Of The Pharmaceutical Domain
Majority (82%) of the CAR-T cell therapies are being developed using autologous T-cells, followed by those using allogeneic cells, derived from the blood of healthy donors. It is worth highlighting that majority of these CAR-T therapies are monotherapies (92%) followed by combination therapies. Additionally, most of the therapies being developed for the treatment of acute lymphoblastic leukemia, B-cell lymphoma, multiple myeloma, non-Hodgkin lymphoma and acute myeloid leukemia using several target antigens which includes CD19, BCMA, CD22, CD20, Meso, and CD7.
A Rise In Partnerships, In The Recent Past, Involving Both International And Indigenous Stakeholders, Validate The Growing Interest In This Domain; Maximum Number Of Such Deals Were Signed In 2021
Since 2015, the partnership activity in this domain has increased significantly. Given that most of the products are commercially available, in recent years, numerous agreements for product evaluation have been inked. Majority of partnerships are licensing agreements followed by R&D agreements (21%); most of the licensing deals (68%) are focused on technology licensing for the development of CAR-T therapies.
Several Investors, Having Realized the Opportunity Within This Upcoming Segment, Have Invested Close To USD 25 Billion
More than 65% of the total funding amount was invested in the last five years. Specifically, in 2022 so far, companies engaged in this domain have collectively raised more than USD 7 Billion, majority of the funding was acquired through venture capital rounds, other equity financing elements and secondary offerings.
Over 6,500 Patents Related to Car-T Therapies Have Been Recently Filed / Granted, Demonstrating The Continued Innovation In This Domain
Majority of the patents related to CAR-T cell therapies have been filed by non-industry players; more than 1,400 such patents were filed in 2021 alone. Additionally, most of the patents in this domain are patent applications (84%), followed by granted patents (13%) and Others (3%).
The Projected Opportunity for Car-T Cell Therapies Is Likely to Be Well Distributed Across Different Segments And Is Estimated To Grow At An Annualized Rate Of 20%, Till 2035
The global CAR-T cell therapy market is estimated to be worth USD XX billion in 2022, and this value is projected to reach around USD XX billion in 2035, growing at a CAGR of 20%, during the period 2022-2035. In the long-term, the overall projected opportunity is likely to be well distributed across key market segments, including target indications, target antigen, key players and geographical regions.
For additional details, please visit
https://www.rootsanalysis.com/blog/car-t-cell-therapies-addressing-key-unmet/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. 4D Bioprinting Market, 2023-2035
2. Non-Viral / Intracellular Drug Delivery Systems Market, 2023-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

8 февраля 2023
Exploring How Artificial Intelligence Is Transforming Digital Pathology
Digital pathology is a transformative technology that has enabled pathologists to make the shift from visual inspection of tissue samples to automated image analysis.
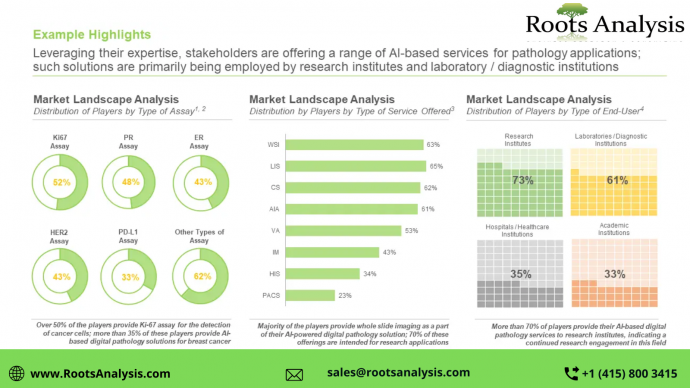
With the advent of AI, pathologists are now benefiting from computerized image analysis software that can draw conclusions and deliver diagnoses in a fraction of the time. Now, pathologists can analyze the RNA / DNA or protein expression of a sample, which is far more accurate than visual analysis. AI is being used to analyze images and extract meaningful information from them, to automate tedious tasks, and to create new ways of diagnosing and treating diseases. Pathologists can rely on AI to extract features from images and classify them into appropriate categories for diagnosis.
Benefits of AI in Digital Pathology
AI is revolutionizing digital pathology by improving accuracy, consistency, and operational efficiency. AI is improving accuracy by enabling pathologists to derive more insights from images and improving the consistency of results by reducing bias. AI-driven analysis is repeatable and consistent, leading to more accurate diagnoses. AI is also improving operational efficiency by automating and optimizing workflows. Pathologists can now rely on AI to manage their workflows, reducing the time spent on tedious tasks.
AI-Based Automation of Pathology Workflows
AI is transforming pathology workflows by automating processes and optimizing collaboration among team members. Pathologists can now leverage AI to manage their workflows, helping to optimize workflow operations. AI can be used to automate processes like image acquisition, data management, and report formatting, as well as recordkeeping tasks.
With AI, pathologists can collaborate more effectively with other team members by sending them notifications on issues, such as abnormal test results or abnormal exam findings. AI can be used to facilitate collaboration between pathologists and other health care professionals, such as researchers and clinicians. Pathologists can send images and findings to researchers for further analysis.
AI-based digital pathology process
Preparation of Tissue Sample
Converting into Virtual Sample / Whole slide imaging (WSI)
Saving a Virtual Slide
Viewing and Editing of Slide
Sharing Data
Reporting Results
AI-Driven Automated Diagnosis & Treatment
New AI technology is allowing pathologists to make diagnoses and prescribe treatments using a machine-generated narrative. AI is being used to analyze images and extract meaningful information from them, enabling pathologists to make more accurate decisions about diagnoses. AI can be used to analyze tissue samples as well as blood and urine samples to derive a more accurate diagnosis.
New AI technology is allowing pathologists to make diagnoses and prescribe treatments using a machine-generated narrative. AI can be used to analyze images and extract meaningful information from them, enabling pathologists to make more accurate decisions about diagnoses. AI can be used to analyze tissue samples as well as blood and urine samples to derive a more accurate diagnosis.
AI in Enhancing Accessibility & Efficiency of Digital Pathology
AI is helping to make digital pathology more accessible, efficient, and reliable. With AI, pathologists can now access more accurate data and insights in a fraction of the time. AI-driven solutions are able to understand and interpret complex data, making them highly accessible and efficient. AI is helping to make digital pathology more reliable by minimizing errors in data analysis. AI is being used to analyze images and extract meaningful information from them, enabling pathologists to make more accurate decisions about diagnoses.
Challenges for AI in Digital Pathology
With the advent of AI, digital pathology is being transformed from visual inspection to molecular analysis. However, there are some challenges associated with AI in digital pathology. The first challenge is the cost of implementation. For organizations to benefit from AI-driven solutions, they have to invest in the technology and hire AI experts to put the technology to use. The second challenge is the lack of understanding of AI by both pathologists and patients. Pathologists are not well-versed with AI and its capabilities, and patients are not aware of the role of AI in pathology, which may lead to inaccurate conclusions.
Conclusion
Digital pathology is transforming the traditional pathology workflow by enabling pathologists to make better, more accurate diagnoses through automated image analysis. With the advent of AI, digital pathology is now able to analyze images and extract meaningful information from them, enabling pathologists to make more accurate decisions about diagnoses. With AI, pathologists can now access more accurate data and insights in a fraction of the time, making it easier for them to make correct decisions about patient care, treatment, and prognosis.
For additional details, please visit
https://www.rootsanalysis.com/blog/how-artificial-intelligence-transforming-digital-pathology/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

7 февраля 2023
The smart pharmaceutical and healthcare labels market is anticipated to grow at a CAGR of 16%, till 2035, claims Roots Analysis
In order to overcome the limitations and challenges related to counterfeit drugs, researchers and industry stakeholders are gradually adopting smart pharmaceutical and healthcare labels, which offer multiple benefits over conventional labels.
Roots Analysis has announced the addition of “Smart Pharmaceutical and Healthcare Labels Market, 2022-2035” report to its list of offerings.
A smart label comprises of printed information, a bar code identifier and a small transponder. This transponder contains a processing chip and an antenna that allows physical objects to communicate with the tag reader (for instance, RFID reader). Smart labels offer several benefits, such as tracing and tracking products in a supply chain, tamper proofing, as well as discouraging counterfeiters from packaging fake drugs. In this context, it is worth mentioning that smart labelling technology can potentially save USD 0.8 - 1.5 million per year, per company, through their applications in inventory management.
Key Market Insights
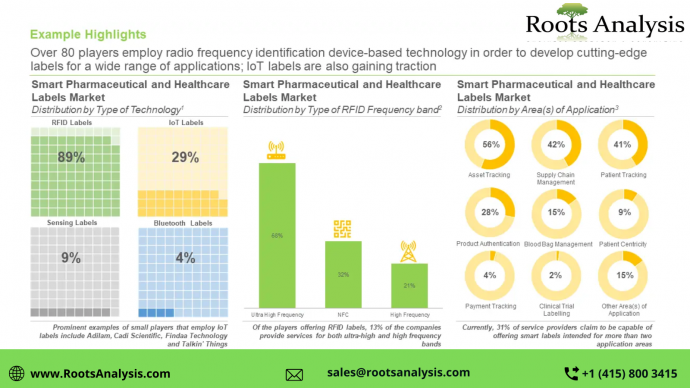
More than 90 companies claim to offer smart pharmaceutical and healthcare labels, globally
This segment of the industry is dominated by the presence of mid-sized (51-500 employees) and small (less than 50 employees) players, which collectively represent more than 75% of the total smart label providers in pharmaceutical and healthcare sectors. Further, ~20% of firms were established post-2010.
400+ patents related to smart pharmaceutical and healthcare labels were filed / granted, since 2015
Based on intellectual property distribution across the world, R&D activity related to smart pharmaceutical and healthcare labels is largely concentrated in North America (over 65%), followed by Europe (10%). In addition, most of the patents have been granted patent (55%), followed by patent applications (44%).
Partnership activity in this domain has increased at a significant pace, between 2018 and 2022
The recent partnerships trend in this market indicates the growing interest of industry stakeholders. It is worth highlighting that majority of the deals were service alliances, representing around 41% of the total number of partnerships signed in the given time period.
North America and Europe are anticipated to capture over 65% of the market share by 2035
The markets in Asia-Pacific and Latin America are anticipated to grow at a relatively faster pace. In the long-term, the aforementioned regions are expected to collectively represent 33% of the total market share. In addition, RFID based smart labels are likely to capture more than 75% of the market share in 2035.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/smart-labels-market/request-sample.html
Key Questions Answered
Who are the leading players engaged in the development of smart pharmaceutical and healthcare labels?
What is the relative competitiveness of different smart pharmaceutical and healthcare labels providers?
Which types of partnership models are most commonly being adopted by stakeholders engaged in this domain?
What are the key strategies that can be implemented by emerging players / start-ups to enter the highly competitive market for smart labels for the healthcare sector?
Which companies are actively filing patents to drive innovation in the field of smart pharmaceutical and healthcare labels?
What are the key challenges within the smart pharmaceutical and healthcare labels market?
What is the current / likely future market size of smart pharmaceutical and healthcare labels?
The financial opportunity within the smart pharmaceutical and healthcare labels market has been analyzed across the following segments:
Type of Technology
RFID
NFC
Other Technologies
Type of packaging
Primary Packaging
Secondary Packaging
Type of Primary Packaging
Vials
Syringes
Cartridges
Ampoules
Bottles
Blister Packs
Type of Secondary Packaging
Boxes
Cartons
Pouches
Key Geographical Regions
North America
Europe
Asia-Pacific
MENA
Latin America
The report includes detailed profiles of key players (listed below) engaged in providing smart pharmaceutical and healthcare labels; each profile features an overview of the developer, details related to smart labels focused product portfolio, recent developments and an informed future outlook:
CCL Industries
Schreiner
Datalogic
Tadbik
SATO
Invengo
Intellhydro Technology
RFiD Discovery
ID Tech Solutions
For additional details, please visit https://www.rootsanalysis.com/reports/smart-labels-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Pharmaceutical Secondary Packaging Market: Industry Trends and Global Forecast, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com

6 февраля 2023
The TCR-based therapy market is projected to grow at an annualized rate of 51%, claims Roots Analysis
Given the recent approval of a TCR-based therapy, industry stakeholders have initiated several R&D efforts focused on evaluating the potential of such therapy candidates against various disease indications
Roots Analysis has announced the addition of “Global TCR-based Therapy Market (2nd Edition), 2022-2035” report to its list of offerings.
Modified T-cell receptor therapy (TCR therapy) is an emerging class of adoptive therapy that employs genetically modified lymphocytes to target specific tumor markers. Growing burden and unaddressed complex treatment needs of cancer patients have paved the way for more effective, safe and versatile alternatives, such as TCR therapies. Driven by Kimmtrak® approval, promising development pipeline and encouraging clinical trial results, the TCR-based therapy market is anticipated to witness significant growth in the mid to long-term
Key Market Insights
Over 190 TCR-based therapies are currently approved / under development
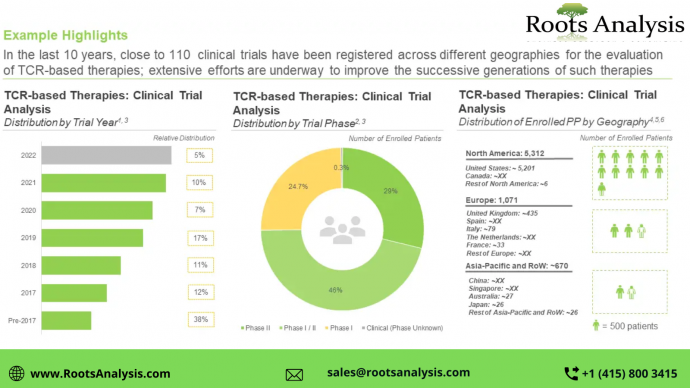
Close to 45% of the aforementioned candidates are being evaluated in clinical trials (phase I and above), while 30% of them are in preclinical stage of development. More than 90% of the therapy candidates, which are being developed to target a range of disease indications, are autologous in nature. Further, NY-ESO-1 and MAGE have emerged as the most popular target antigens.
More than 7,000 patients have been enrolled in nearly 110 clinical trials, worldwide
Clinical research activity, in terms of number of trials registered, is reported to have increased over the last five years. Of the total number of trials, close to 65% are active and still recruiting patients, while close to 26% have already been completed.
Partnership activity within this domain has grown at a CAGR of 27%, between 2015 and 2021
More than 70 agreements have been inked in the last 5 years. Majority of partnership deals signed within this domain were R&D agreements (26%), licensing agreements (21%), and product development and commercialization agreements (16%).
Over 11.2 USD billion has been invested by both private and public investors in this domain, since 2007
Specifically, in 2020 and 2021, industry players raised over USD 2.7 billion. In addition, majority (39%) of the companies primarily received funding through venture capital rounds. Further, around 70% of the total funding instances were reported by players headquartered in the US.
More than 70 patents have been filed / published related to the TCR-based therapies
Close to 85% of the filed / published patents were patent applications, while around 15% were granted patents and others. Of the total granted applications, close to 45% were filed in North America and Europe.
The market is anticipated to grow at a CAGR of 51%, during the period 2022-2035
In 2035, close to 60% of the market revenues are expected to be generated from sales of therapeutics intended for Nasopharyngeal Carcinoma and Multiple Myeloma. Further, therapies targeting for NY-ESO-1 are expected to occupy a larger share (~50%) of the overall market in 2035.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/tcr-based-therapies-market/request-sample.html
Key Questions Answered
What is the growth rate of TCR-based therapy market?
Which region has the highest growth rate in the TCR-based Therapies market?
Who are the leading industry and non-industry players in this market?
How many players are developing TCR-based Therapies?
Which target indication covers the largest TCR-based therapy market share?
What is the partnership and collaboration trend in the TCR-based therapy domain?
What is the current IP landscape of TCR-based therapies market?
The financial opportunity within the TCR-based therapies market has been analyzed across the following segments:
Target Indication(s)
Nasopharyngeal Carcinoma
Multiple Myeloma
Head and Neck Carcinoma
Sarcoma, Melanoma
Acute Myeloid Leukemia
Lung Cancer
Ovarian Cancer
Merkel Cell Cancer
Target Antigen
NY-ESO-1
EBV
gp100
Others
Key Geographical Regions
North America
Europe
Asia Pacific
Latin America
Middle East and North Africa
Rest of the World
The report features inputs from eminent industry stakeholders, according to whom, T-cell immunotherapies are expected to be the next big step in cancer immunotherapy. The report includes detailed transcripts of discussions held with the following experts:
Vincent Brichard (Vice President, Immuno-Oncology, Celyad)
Adrian Bot (Vice President, Scientific Affairs, Kite Pharma)
Victor Lietao Li (Co-Founder and Chief Executive Officer, Lion TCR)
Miguel Forte (Former Chief Operating Officer, TxCell)
The report includes brief profiles of key players (listed below); each profile features an overview of the company, details related to its financial information (if available), details about TCR-based product(s), such as information on type of therapy and current development status, information on technology portfolio (if available), recent developments related to TCR-based therapies and manufacturing capabilities of the players.
Adaptimmune Therapeutics
Alaunos Therapeutics
Bristol Myers Squibb
Cellular Biomedicine Group
Gilead Sciences
GlaxoSmithKline
Immatics
Immunocore
Lion TCR
Takara Bio
Zelluna Immunotherapy
For additional details, please visit https://www.rootsanalysis.com/reports/tcr-based-therapies-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Global T-Cell (CAR-T, TCR, and TIL) Therapy Market (6th Edition), 2022- 2035
2. mRNA Therapeutics and mRNA Vaccines Market (2nd Edition), 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com

3 февраля 2023
DRIVEN BY THE GROWING DEMAND FOR MEDICAL DEVICES, NOVEL COATINGS PROVIDING UNIQUE FEATURES ARE BEING INTRODUCED IN THE MARKET
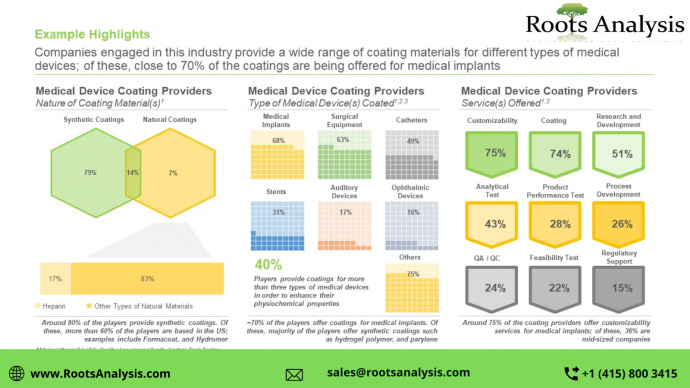
Over the past 50 years, the medical device industry has seen several notable developments. In fact, the use of biomedical devices, such as medical implants, surgical instruments, and prostheses has increased substantially over the time.
Cardioverter defibrillators, prosthetic hips and knees, contact lenses, and cardiac pacemakers are some of the most commonly implanted medical devices, while fixation devices and artificial joints account for about 44% of all medical devices. Despite significant advancements in the design and implantation of medical devices, a number of challenges still persist. Patients, particularly those who are immunocompromised, are at high risk of healthcare-associated infections (HAIs) due to the in-dwelling nature of implanted devices and surgical tools. The risk of infections along with limitations including, implant rejection, osseointegration, degradation and wear, loom over the prosthetic integration. Coatings on biomedical implants can affect this biological interaction between the implant and host, mitigate joint wear, and combine the properties of several materials to enhance device performance as well as reduce the risks associated with invasive medical devices. Medical device coatings also endow properties like lubrication, anti-fouling nature, as well as enhanced durability to the surface of the device. Driven by the surge in demand for various types of medical devices and the need for a wide array of coatings for these devices, the medical device coatings market is anticipated to witness a steady growth in the coming decade.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/medical-device-coatings-market/request-sample.html
One of the key challenges associated with in-dwelling medical devices lies in the fact that they are in constant association with the bodily fluids, therefore, interacting with a wide variety of proteins and other biomolecules. This results in denaturization of the biomaterials over time, reducing the overall functional life of the device. As a counter to this problem, surface modification can be utilized. Generally, protein adsorption and biological interactions are significantly impacted by the biomaterial surface modifications, such as modifying the chemistry of polymers, coefficient of friction, domain layout, and shape. Surface modifications, such as plasma spraying, vacuum depositions, etching, and electro-depositions are some of the most frequently procedures to achieve the necessary alterations on medical device surfaces.
Various medical devices are associated with the risk of infections, cytotoxicity, thrombogenesis, and immune rejection, which has garnered the attention towards appropriate selection of suitable biomaterials and strategies for modifying the medical devices surface. One of the key developments in the area has been made in creating non-fouling / antibacterial surfaces. A breakthrough in this field has been witnessed in graphene coatings developed to elicit antimicrobial properties. As an alternative to the use of silver nanoparticles earlier, the use of graphene in healthcare applications has a promising future, given the fact that graphene would provide more secure antibacterial properties than metal nanoparticles along with cost efficiency. The detailed competitiveness analysis of various medical device coating providers identified during our research, which are based in North America, Europe, and Asia.
Integration of new technologies like advanced laser processing, machine learning, and automation using Artificial Intelligence (AI) also drives the field towards more precise and reproducible coating / surface treatment applications, which can revolutionize the biomedical devices industry.
For additional details, please visit https://www.rootsanalysis.com/reports/medical-device-coatings-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Targeted protein degradation market, 2022-2035
2. Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com