berry cristan
23 февраля 2023
TIL Therapies: A New Paradigm in Cancer Treatment
Till date, several clinical trials have demonstrated the efficacy and therapeutic superiority (over conventional treatment options) of TIL-based therapies.
Modified tumor-infiltrating lymphocytes or TIL therapies have emerged as a viable and potent option to selectively eradicate the tumor population, with minimal side effects. Their tumor-cell killing efficiency is attributed to the fact that they are pre-sensitized to cancer specific antigens.
Ongoing and planned clinical research initiatives in this direction are driven by encouraging results achieved in past trials, which were mostly focused on various hematological cancers and solid tumors. Driven by the ongoing pace of innovation in this field, sufficient financial support from investors and encouraging clinical trial results, the TIL-based therapy market is likely to witness significant growth in the foreseen future.
Current Market Landscape of TIL-based Therapies
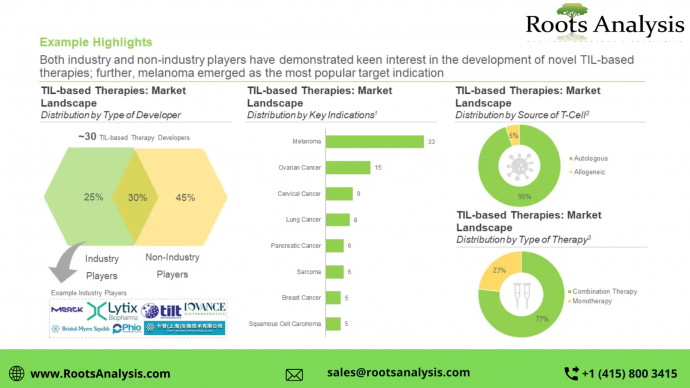
Over 75 TIL immunotherapies are being evaluated across different stages of preclinical / clinical development, either as monotherapies or in combination with other drugs. Both industry and non-industry players have demonstrated keen interest in the development of novel TIL-based cell therapies. Further, melanoma emerged as the most popular target indication in this domain. More than 95% of the therapy candidates that are being developed to target a wide range of disease indications are autologous in nature.
Driven by a promising development pipeline and encouraging clinical trial results, the TIL therapy market is likely to carve out a significant share of the multibillion-dollar cancer immunotherapy market.
Rising Interest in TIL-based Therapies
The growing interest in this field is therapies is reflected from the increase in the partnerships focused on R&D of such therapies. Several investors, having realized the opportunity within this upcoming segment of T-cell immunotherapy, have invested USD 2.7 billion, across 30 instances, since 2013. Further, in the last 10 years, close to 95 clinical trials evaluating TIL-based therapies have been registered across different geographies for the evaluation of TIL-based therapies. Mostly driven by the need for effective treatment options for cancer, the TIL-based therapy pipeline is expected to steadily grow over the coming years.
For additional details, please visit
https://www.rootsanalysis.com/blog/til-based-therapies/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

22 февраля 2023
Viral Clearance and Viral Testing Services
Considering the rising demand for biologics, innovators in the biopharmaceutical industry are constantly exploring avenues that would enable them to overcome the existing technical and operational challenges related to viral-clearance and testing for timely approval of biologics.
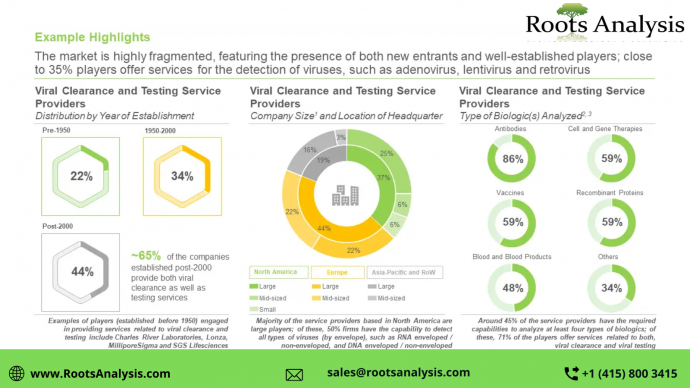
Outsourcing the viral clearance and viral testing studies has emerged as a promising alternative for most of the manufacturers. Despite being one of the crucial steps amongst all the steps involved in the production of biologics, this service is heavily outsourced in the biopharmaceutical industry. Currently, it is estimated that more than 70% of these services are employed by biopharmaceutical and pharmaceutical companies. Service providers across the globe offering this service are large players.
Of these, close to 80% players provide services for both, viral clearance and viral testing of different type of biologics. Of the players have established their facilities in Europe. Within this region, UK has the maximum number of facilities (over 21%), followed by Germany (more than 15%) and France (close to 10%). Patents have been filed / granted over last few years. Of these, over 40% patents are granted by organizations headquartered in the US whereas close to 25% patent applications are filed by organizations headquartered in China.
As projected by Roots Analysis, more than 55% of the share is captured by viral removal, in the viral clearance market. As projected by Roots Analysis, 40% of the share is captured by North America, in this market. Driven by the rising interest in research and development activities and the demand for the viral clearance and viral testing services, the future opportunities and growth associated with this market are anticipated to witness a noteworthy growth in the foreseen future. Specifically, in terms of end-users, the market is anticipated to be driven by biotechnology and pharmaceutical industry.
For additional details, please visit
https://www.rootsanalysis.com/blog/viral-clearance-and-viral-testing-services/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

21 февраля 2023
Artificial Intelligence (AI) In Oncology: Current Scenario and Future Potential
In the last decade, the popularity of AI has grown invariably. It has made a considerable impact in the medical sector and oncologic treatment as well, due to a surge in electronic data, breakthroughs in technological infrastructure and groundbreaking research in deep learning neural networks.
AI has demonstrated potential in improving tumor imaging diagnosis and therapy response evaluation, anticipating clinical outcomes, and accelerating drug development and translational oncology. AI has the potential to revolutionize the oncology sector, by overcoming the existing challenges, by leveraging the power of big data to further improve the cancer treatment. Although AI is already being used in oncology clinical practice, ongoing and increased efforts are required to allow AI to reach its full potential.
AI in Oncology Market – Current Market Landscape
Currently, over 76 industry players worldwide are actively engaged in the development of AI in oncology- based software solutions. The market is characterized by a mix of well-established and small firms. Several industry players involved in the development of AI in oncology- based software solutions are majorly providing these services for cancer diagnosis, along with drug development and drug discovery. Majority of the software providers have their platforms on cloud, for end users which include, hospitals, pharmaceutical companies and research institutes. The growing pipeline and the increasing demand for effective diagnosis of cancer indications at an early stage using AI to prevent malignancies, has spurred the establishment of many companies in the last decades; currently, AI in diagnostics market is dominated by companies based in North America, majority of them being small sized.
Mutually Beneficial Partnerships in Order to Expand Capacities to Keep Pace with the Growing Demand
Several partnerships have been established by various stakeholders engaged in the development of Artificial Intelligence in oncology-based software solutions in the past 5 years; there has been a significant increase in partnership activity in this domain, growing at a CAGR of 36%, during the period 2017-2022.
Surge in Funding Activity in this Domain Foreseeing Lucrative Returns
There has been a steady increase in the funding activity within this domain during the period 2017-2022 which amounted to more than USD 5.9 billion.
High Number of Patents are Suggestive of the Widespread Research in this Domain
Several industry and non-industry players are involved in the development of Artificial Intelligence in Oncology- based software solutions. Over 2,770 patents have been granted / filed by academic and industry stakeholders till date.
Future Evolution of AI in Oncology Market
Owing to the anticipated AI in the oncology sector and given the fact that several new players have entered the domain in the last decade, who are actively collaborating with other industry / non-industry players to expand the global reach of this domain the market opportunity associated with AI in oncology is anticipated to grow at a CAGR of 54%.
For additional details, please visit https://www.rootsanalysis.com/blog/ai-in-oncology/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com


20 февраля 2023
Rising Demand for Medical Devices CROs
Over the past decade, numerous scientific and technological breakthroughs in the medical device CROs industry have resulted in an accelerated pace of research and innovation within this domain.
Further, the demand for advanced and minimally invasive medical devices has been augmented due to the increase in expenditure on healthcare. However, one of the primary challenges faced by this industry is the complex and time-consuming product development lifecycle of a new medical device. Specifically, the clinical stage is exceedingly resource intensive, involving high costs and greater risks.
Outsourcing Necessity for Medical Devices
New advancements have facilitated the development and enforcement of more informed regulatory guidelines and instructions to ensure the safety of medical devices. Subtle differences in regulatory guidelines issues across various geographical regions need to be considered by medical device developers in order to receive approval in different markets. This has further resulted in longer trials, higher costs (due to rise in patient enrollments), and increased time-to-market. In order to overcome the above-mentioned challenges, medical device developers are actively outsourcing their clinical research and associated operations to contract service providers, which are known to have the required capabilities and expertise.
Companies involved in Offering Medical Device CROs
CROs tend to actively strive to enhance / upgrade their respective capabilities and expertise to meet the strict quality and safety specifications, as well as to keep up with any change in medical device-related regulations. This not only enables them to attract more business opportunities from sponsors, but also grants them an edge over competing service provider entities.
Growing Number of Mergers and Acquisitions Validate the Interest in Medical Device CROs Domain
Over the past few years, the merger and acquisitions activity in the medical devices contract research market has increased gradually. It is worth mentioning that in 2021, more than 12 acquisitions have taken place within this industry.
For additional details, please visit
https://www.rootsanalysis.com/blog/rising-demand-for-medical-devices-cros/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

17 февраля 2023
SARM1 Inhibitors: A Novel Approach Against Neurodegenerative Disorders
Presently, several industry and non-industry stakeholders are evaluating SARM1 inhibitors as potential therapeutic agents for the treatment of neurological disorders across various preclinical studies and early stages of clinical development, worldwide.
Given the encouraging research outcomes, the players in this domain have received more than USD 10 million in grants, since 2014, from the various private and public organizations.
Additionally, several patents related to SARM1 targeting therapies have been recently filed / granted, demonstrating the continued innovation in this domain. Driven by the ongoing pace of innovation in this field, increasing R&D activity and promising pre-clinical data, several promising leads are anticipated to be commercially launched over the coming decade and SARM1 targeting therapeutics market is anticipated to witness substantial growth in the mid to long-term.
Current Market Trend
At present, several companies worldwide have taken initiatives to develop SARM1 inhibitors for targeting multiple neurological disorders, including multiple sclerosis and amyotrophic lateral sclerosis. The market is characterized by a mix of well-established and small firms. Currently, their pipeline features both biologics and small molecules drugs, which are being evaluated in preclinical and discovery stages.
Strong Intellectual Property Portfolio
Over the years, the number of patents filed for SARM1 inhibitors has increased gradually; majority of the patents have been filed / granted in 2021. In addition, most of the patents in this domain are patent applications (96%), followed by granted patents (3%).
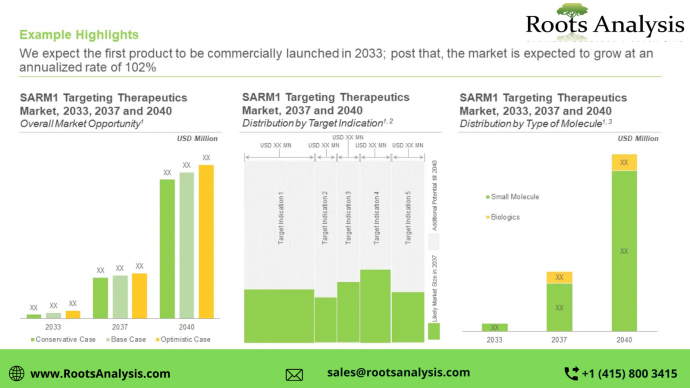
Future opportunities for SARM1 Inhibitors
Currently, there are no potential treatment options that target axonal degeneration associated disorders. Across various studies, SARM1 inhibitors have demonstrated the potential to treat multiple neurodegenerative disorders; once approved, these therapies are likely to capture a sizeable market share in the coming future. Further, we expect the first product to be commercially launched in 2033; post that, the market is expected to grow at an annualized rate of 102%.
For additional details, please visit
https://www.rootsanalysis.com/blog/sarm1-inhibitors-market/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com