berry cristan
18 января 2023
The global digital twins market is projected to grow at a CAGR of 30% till 2035, claims Roots Analysis
Given the potential of digital twins to replicate the physical world in a digital layout, as well as their ability to provide real-time outputs, while gathering constant inputs from the real-world, such products have generated significant interest within the healthcare domain
Roots Analysis has announced the addition of “Digital Twins Market, 2022-2035” report to its list of offerings.
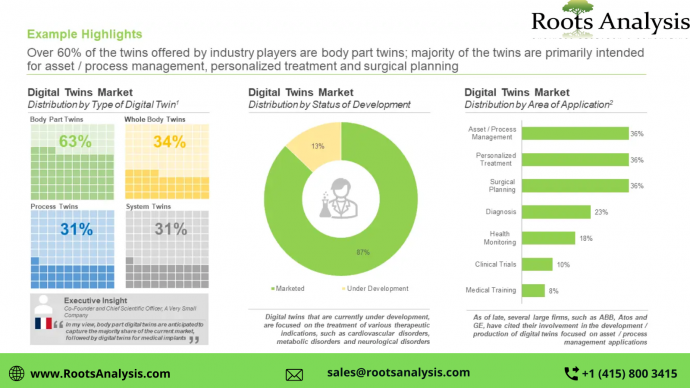
Since the first application of the digital twin concept for manufacturing in 2002, it has emerged as a crucial component for companies to achieve higher efficiency in their production processes. According to the US-based Digital Twin Consortium, digital twins hold the potential to reduce clinical trial expenditure, turnaround times, staffing expenses and plan medical intervention. Digital twin technology companies are currently engaged in the development of products which are intended for numerous applications, such as asset / process management, personalized treatment and surgical planning.
Key Market Insights
Over 35 digital twins are presently available in this market space
More than 60% of the digital twins offered by industry players are body part twins; these products are primarily intended for asset / process management, personalized treatment and surgical planning. Further, majority of the products that are currently under development are focused on the treatment of indications, such as cardiovascular disorders, metabolic disorders, and neurological disorders.
More than 30 players currently claim to be engaged in the digital twin domain
Majority of the players engaged in this industry are small firms (11-50 employees, 37%), followed by very large players (10,000+ employees, 22%), large players (501-10,000 employees, 19%), mid-sized players (51-500 employees, 13%) and very small companies (2-10 employees, 9%). Additionally, nearly 70% of the developers are based in Europe, followed by those based in North America (28%).
Partnership activity for digital twins witnessed a CAGR of over 15%, in the past three years
More than 50% of the partnerships have been signed since January 2020; of these, four partnerships were inked in the year 2022. Technology integration agreements have emerged as the most popular (30%) type of partnership model in the market, followed by technology utilization agreements (23%).
Nearly USD 6 billion invested across the globe to advance digital twins focused initiatives
A steady increase has been observed in funding activity since 2020, with the maximum number of instances (10) being reported in the year 2021. In fact, more than 90% of the total investment (in terms of the amount invested) was made in the last two years alone (2021- 2022). Of the amount raised in 2022, about USD 4.2 billion was contributed by the IPO of Babylon.
Berkus start-up valuation analysis has been performed to evaluate start-ups in this domain
The framework enables valuation of start-ups, based on several key success / risk factors . The key factors are assigned monetary values based on the qualities / risks possessed by a particular player. At the end, all valuations were added to calculate the final valuation of the company.
Players based in North America are anticipated to capture nearly 30% of the market share by 2035
The overall digital twin market is expected to be primarily driven by the growing interest towards automation and prognostic systems. The estimates in our report suggest that digital twins intended to treat cardiovascular disorders are expected to hold 32% share of the market in 2035. It is interesting to mention that market for digital twins intended to serve pharmaceutical companies and medical device manufacturers, collectively, hold over 60% of the market share in 2035.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/digital-twins-market/request-sample.html
Key Questions Answered
Who are the leading players engaged in the development of digital twins for the healthcare domain?
Which type of digital twin is most commonly offered by developers engaged in this market space?
What is the relative competitiveness of different players engaged in the digital twins domain?
What is the likely valuation of start-ups involved in the development of digital twins for the healthcare sector?
What is the present and likely future demand for digital twins in the overall healthcare sector?
What are the anticipated future trends related to digital twins in the healthcare domain?
How is the current and future opportunity likely to be distributed across key market segments?
The financial opportunity within the digital twins market in the healthcare domain has been analyzed across the following segments:
Therapeutic Area
Cardiovascular Disorders
Metabolic Disorders
Orthopedic Disorders
Other Disorders
Type of Digital Twin
Process Twins
System Twins
Whole Body Twins
Body Part Twins
Area of Application
Asset / Process Management
Personalized Treatment
Surgical Planning
Diagnosis
Other Applications
End Users
Pharmaceutical Companies
Medical Device Manufacturers
Healthcare Providers
Patients
Other End Users
Key Geographical Regions
North America
Europe
Asia
Latin America
Middle East and North Africa
Rest of the World
The research includes profiles of key players (listed below); each profile features a brief overview of the company, details related to its recent developments (including funding and collaborations) and an informed future outlook.
Babylon
ExactCure
ImmersiveTouch
Navv Systems
ThoughtWire
Unlearn.AI
For additional details, please visit https://www.rootsanalysis.com/reports/digital-twins-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Flow Cytometry Service Market, 2022-2035
2. Gene Editing beyond CRISPR Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact Information
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com

17 января 2023
THE INTRICACIES ASSOCIATED WITH CELL THERAPY MANUFACTURING HAVE PAVED THE WAY FOR NOVEL AUTOMATION TECHNOLOGIES
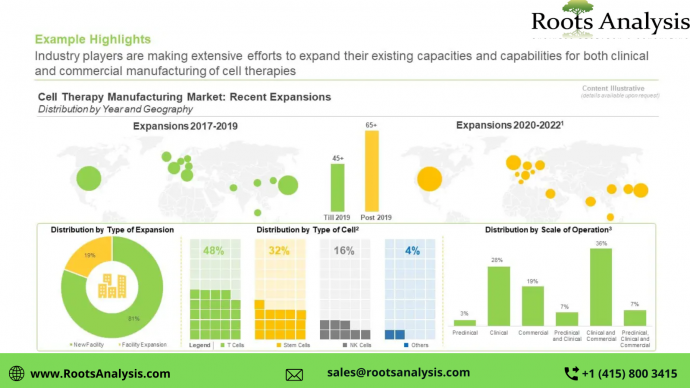
Over the years, several advanced and innovative automation tools and technologies have been developed; these have been demonstrated to hold the potential for significant reduction in the cost associated with the manufacturing of advanced therapy medicinal products, thereby, making such products more affordable.
Considering the vast potential of cellular therapies in the treatment of rare disorders and sufficient body of evidence validating the clinical benefits / therapeutic potential of this complex class of biologic drugs, cell therapies have garnered considerable attention of players engaged in the healthcare industry, in the past few years.
The focus of stakeholders has now shifted to optimizing the cell therapy manufacturing process. It is important to mention that we have forecasted the evolution of the overall cell therapy manufacturing market, focusing on T-cell immunotherapies, dendritic cell therapies, NK cell therapies and stem cell therapies. Considering the sufficient body of evidence validating the clinical benefits / therapeutic potential of this complex class of biologic drugs, the focus of stakeholders has now shifted to optimizing the cell therapy manufacturing process.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/285/request-sample.html
One such emerging concept, namely GMP-In-A Box, offers several advantages, including increased throughput, decreased idle time between batch runs and reduced manual labor. However, the delicate nature of steps involved in the cell therapy production process is known to hinder the overall automation process. Further, the lack of specialized infrastructure and limited expertise available in this domain are some of the known challenges impacting the growth of this segment.
Despite the challenges associated with the development and production of such therapies, we believe, that the benefits offered by them outweigh the hurdles. They are likely to serve as important drivers of the industry’s growth. Efforts to introduce automation technologies in cell therapy manufacturing are underway, and if implemented successfully, can significantly help in the elimination of human intervention and reduce the risk of contamination. As a consequence, it is likely to result in a marked increase in product consistency, ensure the maintenance of sterility, and decrease the production time and cost. In a nutshell, cell therapies are expected to soon represent one of the prominent therapeutic options within the mainstream healthcare.
For additional details, please visit https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com

16 января 2023
The liposome development and manufacturing services market is anticipated to grow at a CAGR of nearly 10% till 2035, claims Roots Analysis
Driven by the development of advanced methods for preparation and characterization of liposomes, and the application of these molecules in therapeutics and diagnostics, the demand for liposomes is expected to rise in the coming years.
London
Roots Analysis has announced the addition of “Liposome Manufacturing Market, 2022-2035” report to its list of offerings.
Despite the growing interest in liposome-based therapeutics and diagnostics, the development and manufacturing of liposomes is associated with several challenges, including complex manufacturing processes, huge capital investments, inadequate clinical grade production and GMP compliant industrial scale-up, lack of facilities with necessary infrastructure, as well as concerns related to storage and stability. In order to deal with the aforementioned challenges, a number of pharmaceutical companies have demonstrated the preference to outsource their respective liposome development and manufacturing operations to specialized service providers.
Key Market Insights
More than 70 companies are currently offering services related to liposome development and manufacturing, globally
Majority (74%) of the service providers offer formulation development services for liposomes. This is followed by players offering analytical method development (65%), liposome contract manufacturing (64%), and process development (61%) services.
Close to 6,100 articles focused on liposomes, have been published in reputed scientific journals, since 2017
More than 50% of the articles focused on liposomes were published post-2019. Popular journals that have published multiple articles include International Journal of Pharmaceutics, Journal of Controlled Release, and Pharmaceutics.
More than 800 clinical trials have been registered for the evaluation of liposome-based therapeutics, worldwide
The clinical research activity, in terms of number of trials registered, is reported to have increased at a CAGR of 17%, during the period 2017-2021. Of the total number of trials registered, 60% have already been completed, while 26% of the studies are actively recruiting participants.
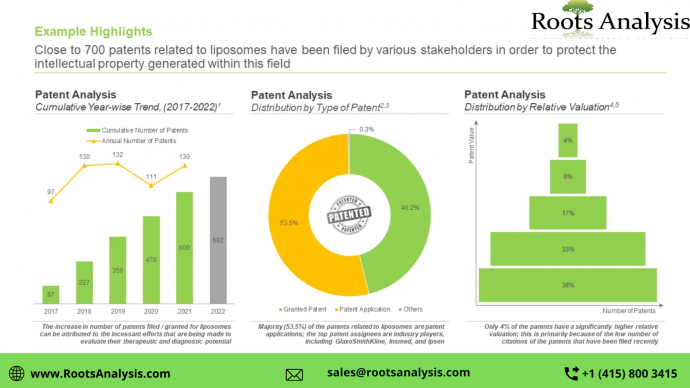
Nearly 700 patents related to liposomes have been filed / granted, since 2017
Owing to the increase in research and development efforts led by several industry and non-industry players engaged in this domain, close to 55% of patent applications have been filed post-2016. It is worth noting that 61% patents related to liposomes were filed / granted in the US alone.
Over 110 global events related to liposomes were organized in the past couple of years
Majority (63%) of the events related to liposomes, were organized virtually in order to comply with the guidelines in the ongoing COVID-19 pandemic. Further, the agendas of the events organized post-2020 include discussions on the applications, and recent advancements in technologies associated with liposomes.
Go / No-Go framework can be used to assist players in the crucial outsourcing decision-making process
The framework enables evaluation of the current capabilities of liposome-based therapeutic developers, based on 4+ parameters, including trial phase, target therapeutic area, location of trial(s), patient enrollment and willingness to outsource.
North America is anticipated to capture over 40% of the global market share for liposome development and manufacturing services, by 2035
In terms of type of product formulation, the therapeutic formulations of liposomes (71%) are anticipated to capture the highest share, this trend is unlikely to change in the foreseen future. Further, based on end users, majority of the revenue share (76%) of the overall market is likely to be driven by pharmaceutical and biotechnology industry, by 2035.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/liposome-manufacturing-market/request-sample.html
Key Questions Answered
Who are the key players offering liposome development and manufacturing services?
What is the evolving trend of publications focused on liposomes?
How many patents, related to liposomes, have been filed / granted in the past few years?
Which companies are actively involved in conducting clinical trials for liposomes?
What are the key agenda items being discussed in various global events / conferences related to liposomes?
Which factors are likely to influence the decision of liposome manufacturing being done in-house or outsourced?
How is the current and future market opportunity related to liposome development and manufacturing services, likely to be distributed across key market segments?
The financial opportunity within the liposome development and manufacturing services market has been analyzed across the following segments:
Type of Product Formulation
Therapeutic
Nutraceutical
Scale of Operation
Discovery / Research
Preclinical
Clinical
Commercial
End User
Pharmaceutical and Biotechnology Industry
Cosmetic Industry
Food Industry
Agricultural Industry
Academics
Other Industries
Key Geographical Regions
North America
Europe
Asia-Pacific
Middle East and North Africa
Latin America
The research also includes detailed profiles of the key players (listed below) offering services for liposome development and manufacturing; each profile features an overview of the company, its financial information (if available), details on service portfolio, recent developments and an informed future outlook.
Baxter BioPharma Solutions
Charles River Laboratories
Evonik
Fresenius Kabi
Fujifilm
GEA
Intertek
For additional details, please visit
https://www.rootsanalysis.com/reports/liposome-manufacturing-market.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Biologics Fill Finish Services Market (3rd Edition), 2022-2035
2. Bioavailability Enhancement Technologies and Services Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

13 января 2023
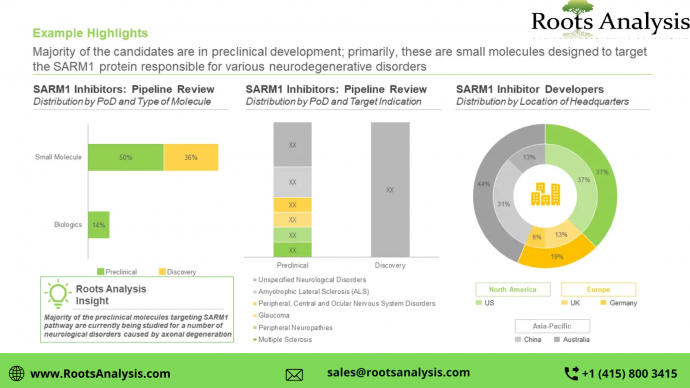
The SARM1 targeting therapeutics market is projected to grow at a CAGR of 102.1%, during the period 2033-2040, claims Roots Analysis
SARM1 inhibitors, having demonstrated the ability to prevent axonal degeneration, are being evaluated for the treatment of a number of neurodegenerative disorders. Once approved, these therapies are likely to capture a significant market share
Roots Analysis has announced the addition of “SARM1 Inhibitors Therapeutics Market, 2022 – 2040” report to its list of offerings.
Presently, several industry and non-industry stakeholders are evaluating SARM1 inhibitors as potential therapeutic agents for the treatment of neurological disorders across various preclinical studies and early stages of clinical development, worldwide. With the ongoing pace of innovation in this field, increasing R&D activity and promising pre-clinical data, several promising leads are anticipated to be commercially launched over the coming decade and SARM1 targeting therapeutics market is anticipated to witness substantial growth in the mid to long-term.
Key Market Insights
Presently, several SARM1 targeting therapy candidates are being developed by various industry players
About 65% of the pipeline candidates are currently being evaluated in the preclinical stages of development, followed by those currently in the discovery stage (35%). Further, it is worth mentioning that close to 85% of the SARM1 inhibitors are small molecules.
Currently, a number of companies claim to be engaged in the development of SARM1 inhibitors
The SARM1 inhibitors market is dominated by the presence of large companies (81%), followed by small players (19%). In addition, around 20% of the players were established post-2010.
120+ articles related to SARM1 inhibitors have been published between 2011 and 2022
Majority (81%) of the articles published in this domain were research papers, followed by review papers (12%). It is important to note that more than 70% of the total number of articles were published post-2018.
Over 30 grants have been awarded for research related to SARM1 inhibitors, since 2014
Close to 40% of the total amount was awarded under the R01 mechanism (which supports research projects). Further, genetics and neurology emerged as the key research departments, having received 34% of the total grants, each.
70+ patents related to SARM1 targeting therapeutics have been filed / granted till 2022
Over the years, the number of patents filed for SARM1 inhibitors has increased gradually; majority of the patents have been filed / granted in 2021. Most of the patents in this domain are patent applications (96%), followed by granted patents (3%).
North America is anticipated to capture more than 65% share of the market, by 2040
By 2040, the market revenues are likely to be driven by the sales of small molecules (95%) being developed as SARM1 inhibitors. Further, sales of therapeutics targeting multiple sclerosis are likely to contribute to a majority share (~40%) of revenues, in the long term.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/sarm1-inhibitors-market/request-sample.html
Key Questions Answered
Who are the leading players engaged in the development of SARM1 targeting therapeutics?
What is the evolving trend of publications focused on SARM1 targeting therapeutics?
How is the intellectual property landscape in this field likely to evolve in the foreseen future?
What are the recent developments and strategic initiatives undertaken by players engaged in this market space related to the research and development of SARM1 targeting therapeutics?
What are the key value drivers that are likely to influence the evolution of this upcoming market?
How is the current and future market opportunity likely to be distributed across key market segments?
The financial opportunity within the SARM1 targeting therapeutics market has been analyzed across the following segments:
Type of Molecule
Small Molecules
Biologics
Target Indication
Multiple Sclerosis
Peripheral, Central and Ocular Nervous System Disorders
Peripheral Neuropathies
Glaucoma
Amyotrophic Lateral Sclerosis
Drug Developers
Company 1
Company 2
Company 3
Drug Candidates
Drug Candidate 1
Drug Candidate 2
Drug Candidate 3
Drug Candidate 4
Drug Candidate 5
Drug Candidate 6
Key Geographies
North America
Europe
The research includes detailed profiles of key players (listed below); featuring an overview of the company, financial information (if available), details related to its product portfolio, patent portfolio, recent developments (including partnerships and collaborations) and an informed future outlook.
Disarm Therapeutics
Nura Bio
Washington University
For additional details, please visit
https://www.rootsanalysis.com/reports/sarm1-inhibitors-market.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. RNAi Market: Therapeutics and Technologies (Focus on siRNA, miRNA, shRNA and DNA) (3rd Edition): Industry Trends and Global Forecasts, 2022-2035
2. Global Therapeutic Vaccines Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

12 января 2023
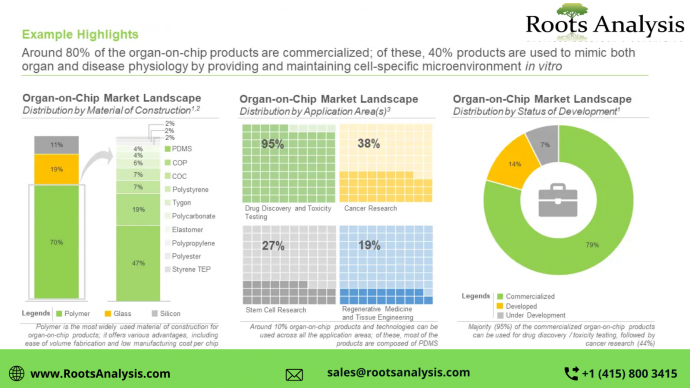
The organ-on-chip market is anticipated to grow at a CAGR of 21.3% till 2035
In order to overcome the limitations and challenges related to animal testing, researchers and industry stakeholders are gradually adopting organ-on-chip products and technologies, which offer multiple benefits over conventional systems.
Roots Analysis has announced the addition of “Organ-on-Chip Market, 2022-2035: Focus on Products and Technologies” report to its list of offerings.
With the introduction of FDA Modernization Act in 2021, the use of animal models for preclinical testing is being discouraged in the pharmaceutical industry. Several players have opted to modernize their conventional testing models with organ-on a chip products and technologies. These novel products have the potential to transform the drug discovery process by simulating the human physiological and functional environment on a microfluidic system. In addition, these organ-on-chip models not only reduce the need for animal testing model but are also capable of speeding-up the research and precise evaluation of drug toxicity and cellular responses.
Key Market Insights
Around 145 organ-on-chip products and technologies are offered by 45+ players
Nearly 67% of the aforementioned products and technologies are commercialized; of these, 93% products and technologies are intended for drug discovery and toxicity testing related applications, followed by cancer research (44%), stem cell research (32%) and tissue engineering and regenerative medicine (21%).
420+ patents related to organ on a chip products and technologies were filed / granted, since 2017
Based on the intellectual property distribution across the world, R&D activity related to organ on a chip technology and product is largely concentrated in China (over 30%), followed by the US (24%). The majority of the patents in this domain were filed by non-industry players (55%).
More than 345 grants have been awarded to support the ongoing research for organ-on-chip models
Grants worth USD 167 million have been awarded to various companies / organizations working in this domain till July 2022. Further, the number of grants awarded to stakeholders in this domain (in the US) has continuously increased between 2019 and 2021. Around 20% of the grants were funded by the National Center for Advancing Translational Sciences.
Partnership activity in this domain has increased at a significant pace, between 2017 and 2022
The maximum number of partnerships were established in 2020 and 2021 (21, each), indicating a recent rise in the interest of players engaged in this domain. It is worth highlighting that the majority of the deals were technology / product utilization agreements, representing around 30% of the total number of partnerships signed in the given time period.
Amount worth over USD 680 million was invested by both private and public investors in this domain, since 2017
Of the total amount invested, around USD 385 million was raised through grants / awards, representing around 55% of the overall funding activity in this domain. Further, 13 instances of venture capital financing were also reported, wherein players collectively raised more than USD 215 million.
North America and Europe are anticipated to capture over 75% of the market share by 2035
The market in Asia-Pacific and Rest of the World are anticipated to grow at a relatively faster rate to occupy overall 24% of the total market. In addition, it is worth noting that organ(s) based models are likely to capture more than 70% of the market.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/organ-on-a-chip-market/request-sample.html
Key Questions Answered
Who are the leading players engaged in the development of organ-on-chip products and technologies?
What are the different application areas of organ-on-chip products and technologies?
Which are the key geographies where organ-on-chip developers are located?
How has the intellectual property landscape of organ-on-chip evolved over the years?
Which partnership models are commonly adopted by stakeholders in the organ-on-chip domain?
What are the investment trends and who are the key investors actively engaged in the organ-on-chip domain?
How is the current and future opportunity likely to be distributed across key market segments?
The financial opportunity within the organ-on-chip market has been analyzed across the following segments:
Type of Product
Organ(s) based
Disease(s) based
Type of Application Area
o Drug Discovery and Toxicity Testing
o Cancer Research
o Tissue Engineering and Regenerative Medicine
o Stem Cell Research
Purpose
o Research Use
o Therapy Development
Key Geographical Regions
o North America
o Europe
o Asia-Pacific
o Rest of the World (RoW)
The opinions and insights presented in the report were influenced by discussions held with senior stakeholders in the industry. The report features detailed transcripts of interviews and surveys held with the following industry players:
Michael Shuler (President, Hesperos)
Matt Dong-Heon Ha (Chief Executive Officer, EDmicBio)
Jelena Vukasinovic (Chief Executive Officer, Lena Biosciences)
Maurizio Aiello (Chief Executive Officer, react4life)
Michele Zagnoni (Chief Executive Officer, ScreenIn3D)
Pierre Gaudriault, (Chief Business Development Officer, Cherry Biotech)
The report includes detailed profiles of key players (listed below), engaged in the development of organ-on-chip products and technologies; each profile features an overview of the developer, product portfolio and recent developments, and an informed future outlook:
BEOnChip
Dynamic42
Emulate
MIMETAS
SynVivo
TissUse
uFluidix
For additional details, please visit https://www.rootsanalysis.com/reports/organ-on-a-chip-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. 3D Cell Culture Market (4th Edition): Industry Trends and Global Forecast, 2022-2035
2. Artificial Intelligence in Oncology Market: Industry Trends and Global Forecast, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com