30 января 2023
DIGITAL THERAPEUTICS - THE INCREASING REGULATORY SUPPORT HAS FACILITATED THE ESTABLISHMENT OF A STANDARD DEVELOPMENTAL PATHWAY FOR THESE SOLUTIONS
Digital therapeutics are clinically validated applications / software / online programs that have demonstrated the capability to facilitate positive outcomes when used in the prevention / treatment / management of diseases / clinical conditions.
Digital Therapeutics, popularly known as DTx, represent a digital health solution which delivers medical interventions directly to the patients in order to treat, manage and prevent a disease. These therapeutics are designed to engage patients in personalized treatment or disease prevention programs, through mediating behavioral or psychological modifications, providing motivational support and inculcating healthy lifestyle changes.
Several organizations have undertaken diverse initiatives in the field of digital therapeutics to support its growth as a new frontier in the healthcare sector. A number of organizations focused on effectively monitoring and promoting the potential of digital therapeutics to be used as a part of strategies to improve the population health have also been established. These organizations include:
Digital Therapeutics Alliance (DTA)
Personal Connected Health (PCH) Alliance
Centers for Disease Control and Prevention (CDC)
Health Insurance Portability and Accountability Act (HIPAA)
National Health Service (NHS)
United States Food and Drug Administration (USFDA)
To request a sample copy / brochure of this report, please visit this
https://www.rootsanalysis.com/reports/208/request-sample.html
Given the current activity in this domain and the growing demand for such solutions, the digital therapeutics market is likely to grow at a healthy pace over the next decade. Traversing a digital therapeutic from the R&D stage to the market is a long process. The various developmental stages involved in this process have been discussed in detail in the following sections.
Discovery and Preclinical Phase: The discovery phase involves the identification of a novel digital therapeutic intervention. At this stage, researchers publish their work in academic journals and continue to investigate the potential applications of their digital solutions in disease treatment / management.
Clinical Trials and Validation: This phase involves the conduct of proper clinical trials to validate the claims made by a digital therapeutic solution provider, and to evaluate its potential in a real-world setting. It includes testing of the digital therapeutics software / hardware on a specific patient population. In case of clinical studies, health outcomes are measured and tracked through data driven insights provided by the software. Disease specific improvements (post application / implementation of the intervention) are also tracked to evaluate the performance of a product. There are multiple challenges associated with conducting clinical trials for digital therapeutics. Firstly, technologies are known to change rapidly and there is a very high probability for a software to undergo upgrades / improvements over the duration of a clinical trial. As a result, there are technical issues in storing and updating patient data. Secondly, digital interventions cannot be studied in a double-blind manner, because the investigator is always aware of whether a trial subject is in the control group or being treated with the intervention under evaluation. Finally, at present, there is less structure and guidelines available, and as a result meaningful and conclusive insights are difficult to be drawn from such trials.
Negotiations with Insurance Providers / Payers: Post the successful completion of clinical studies, developer companies generally tend to avail reimbursement opportunities for their products in order to promote the use of their proprietary solutions and provide financial benefits to patients / consumers. As is the case with pharmacological interventions, reimbursement plans for these products can be achieved based on the outcomes of clinical trials and depending on the USFDA’s (or the concerned regulatory authority of a particular region) clearance. A number of health insurance providers, such as Medicare and Humana, are actively working to include digital therapeutics as a part of health insurance coverage plans for patients suffering from chronic diseases.
Distribution and Marketing: The pharmaceutical and medical device distribution / marketing system is an established network with well-defined channels through which manufacturers can reach the end-users of their products. Product developers in this domain are presently looking to create a distribution network to sell their offerings in the market via both B2B (healthcare providers, regulators and payers) and B2C (customer) models.
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/digital-health-market/208.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

27 января 2023
AI-BASED DIGITAL PATHOLOGY GAINS INTEREST DUE TO AUTOMATION, DIGITIZATION OF HEALTHCARE, SHORTAGE OF PATHOLOGISTS, AND INCREASED DEMAND FOR PATHOLOGY SERVICES
In recent years, advancements in technology and emphasis on precision medicine have recently paved the path for the development of artificial intelligence (AI) based digital pathology techniques for quantitative and qualitative assessment of samples.
Specifically, the process of AI-based digital pathology allows scanning of slides via computer monitors, by replacing the conventional microscopic approaches. Further, by converting glass slides to images, samples can be transmitted from diagnostic centers to pathologists within a fraction of time. Considering the rising popularity and demand for such solutions in the healthcare and research industry, and the ongoing efforts of AI-powered digital pathology solution providers / AI pathology solution providers to further improve / expand their respective portfolios, we believe that the AI-based digital pathology market / AI Pathology Market is likely to evolve at a steady pace, till 2035.
To request a sample copy / brochure of this report, please visit this
https://www.rootsanalysis.com/reports/ai-based-digital-pathology-market/request-sample.html
The steps involved in the usual workflow of AI-based digital pathology process.
Preparation of Tissue Sample: This process is very similar to the conventional approach. A pathologist examines a biopsy to determine its color, size and consistency. At this point, the specialist can detect symptoms of malignancy and select which areas of a specimen should be inspected under the microscope. Further, the chosen region is prepared by following multi-step processes, such as treatment of the tissue with chemicals in order to maintain its structure, mounting the specimen on a glass slide, staining to improve contrast and protecting the tissue with coverslips.
Converting into Virtual Sample / Whole slide imaging (WSI): WSI or virtual microscopy is a technique that is used to enable digital pathology. Its central component, which is a WSI scanner, captures a picture of the glass slide and generates a precise electronic replica known as a virtual slide. It is worth noting that virtual slides, unlike glass slides, are easy to replicate, save, categorize and distribute. Furthermore, they may be linked to electronic health records, thereby, providing a complete picture of a patient's health.
Saving a Virtual Slide: The scanner pre-processes the virtual slide automatically and stores it to on-premises or cloud storage. In order to minimize the file size, a compression approach is frequently employed before saving the slide.
Viewing and Editing of Slide: In the digital process, instead of using a traditional microscope, a pathologist uses a computer display to analyze enlarged tissue samples. A slide viewing and management software is used to zoom out a tissue segment and observe its smallest features. In addition, this software allows the pathologist to view the slide from different angles, add annotations and even compare multiple images at one time.
Sharing Data: Using specialized digital pathology software applications, slides are converted to an electronic format, thereby, allowing them to be exchanged using the internet. These slides can be shared to gain a second opinion, as well as with patients, research facilities and other stakeholders.
Reporting Results: Some image viewing systems provide reporting capabilities. However, this work is often accomplished by enabling interaction with the laboratory information or laboratory information management system (LIS/LIMS) and hospital information system (HIS).
The digital revolution of pathology is projected to accelerate in the coming years, considering multiple growth drivers, including growing number of laboratories adopting high throughput digital scanning and software technologies to assist diagnostic practice. In addition, factors, including shortage of skilled pathologists in remote areas, increasing pathology workloads due to ageing populations, higher rate of cancer screening programs, rising complexity of pathology testing and time constraints, and requirement for pathology labs to outsource expertise in the field, also contributes significantly towards the need for AI-based digital pathology solutions.
For additional details, please visit
https://www.rootsanalysis.com/reports/ai-based-digital-pathology-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com

25 января 2023
The dental 3D printing market is anticipated to grow at a CAGR of 15.1% during the period 2023-2035, claims Roots Analysis
The growing burden of dental diseases and the challenges associated with the conventional dental product manufacturing methods has significantly increased the adoption of 3D printing in the dental industry
Roots Analysis has announced the addition of “Dental 3D Printing Market, 2023 – 2035” report to its list of offerings.
The popularity of 3D printing technology within the dental industry can be attributed to its ability to print high quality / accurate dental products, including crowns, bridges, dentures, dental implants and surgical guides. Driven by the overall growth of the additive manufacturing (3D printing) industry, and wide adoption of 3D printers in the dental sector, the dental 3D printing market is anticipated to witness a steady growth in the foreseen future.
Key Market Insights
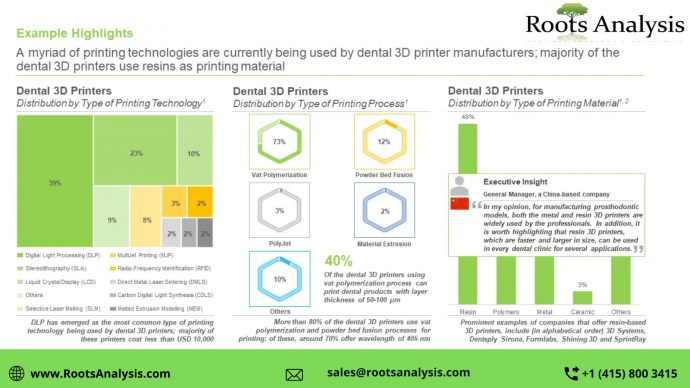
Currently, around 230 dental 3D printers are available for use across wide range of applications
Majority (38%) of the dental 3D printers use digital light processing (DLP) as printing technology, followed by those using stereolithography (SLA) technology (21%). Further, close to 75% of the dental 3D printers can produce crowns / bridges / dentures, followed by those manufacturing working models (61%) and surgical guides (59%). It is worth highlighting that around 55% of the dental 3D printers currently available in the market have a price range below USD 10,000.
More than 80 companies claim to offer dental 3D printers, across the world
Majority of the firms (46%) engaged in this domain are mid-sized players, followed by large (32%) and small firms (22%). It is worth highlighting that, more than 50% of the companies in this domain have been established post-2010. Further, around 85% of the dental 3D printer manufacturers are based in Asia-Pacific and Europe, followed by those headquartered in North America (16%), and within the Asia-Pacific region, China emerged as the most prominent hub, featuring the presence of maximum number of players (62%).
Partnership activity has grown at an annualized rate of over 70%, between 2018-2022
Maximum number of partnerships were established in 2021 (56) and 2022 (44), indicating the recent surge in the partnership activity in this domain. Majority of these (26%) were product integration agreements. Further, most of the intercontinental as well as intracontinental deals have been inked by the players based in North America.
500+ patents related to dental 3D printing have been granted / filed between 2019 and 2022
R&D activity related to dental 3D printing is largely concentrated in Asia-Pacific, considering the fact that 50% of the total number of patents were filed in this region. It is worth highlighting that, within the Asia-Pacific region, China emerged as the most active country with ~230 filed patents. In addition, most of the patents in this domain are patent applications (65%).
North America and Europe are anticipated to capture around 75% share of the market by 2035
In addition, the market in Asia-Pacific is likely to grow at a relatively faster pace (with CAGR of 16.3%) in the long term. Further, in 2035, based on type of printing technology, majority of the revenue share (64%) of the overall market is likely to be driven by vat polymerization technology. Further, in terms of application area, prosthodontics currently holds largest share (55%) of the market.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/dental-3d-printing-market/request-sample.html
Key Questions Answered
How is 3D printing used in the dental industry?
How many dental 3D printers are currently available in the market?
Which 3D printers are best for dentistry?
What is the growth rate of the dental 3D printing market?
Which region captures the largest share of the dental 3D printing market?
Which printing technology is widely used in the dental 3D printers?
Which application area currently holds the largest share in the dental 3D printing market?
What are the partnerships and collaborations trend in the dental 3D printing domain?
How many patents have been filed / granted related to dental 3D printing in the recent years?
The financial opportunity within the dental 3D printing market has been analyzed across the following segments:
Type of Printing Technology
VAT Polymerization Technology
Powder Bed Fusion Technology
Polyjet Technology
Metal Extrusion Technology
Other Technologies
Application Area
Prosthodontics
Orthodontics
Dental Implants
Other Applications
Type of Printing Material
Resins
Plastics
Metals
Metals
Other Material
Key Geographical Regions
North America
Europe
Asia-Pacific
Latin America
Middle East and North Africa
Rest of the World
The research also includes detailed profiles of key players (listed below) featuring a brief overview of the company, details related to dental 3D printer portfolio, recent developments, and an informed future outlook.
3D Systems
Asiga
BEGO
Carbon
Digital Wax Systems (DWS)
Formlabs
Prodways
Rapid Shape
SprintRay
Stratasys
For additional details, please visit
https://www.rootsanalysis.com/reports/dental-3d-printing-market.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. Medical Devices Contract Research Organizations Market (3rd Edition): Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com

24 января 2023
The biologics fill / finish services market is projected to grow at a CAGR of 8%, till 2035, claims Roots Analysis
A number of biopharmaceutical companies are outsourcing their biologics fill / finish operations, thereby, prompting stakeholders engaged in this domain to undertake several expansion initiatives, to cater to the needs of clients around the globe.
Roots Analysis has announced the addition of “Biologics Fill Finish Services Market (3rd Edition), 2022-2035” report to its list of offerings.
Given the growing pipeline of biologics, lack of technical expertise, and huge capital investment in the installation of fill / finish equipment, a number of drug manufacturers are turning to contract service providers in order to ensure the development of quality drug products. This evident surge in the demand for biologics fill / finish services has presented lucrative opportunities for service providers having necessary fill / finish capabilities.
Key Market Insights
More than 175 service providers claim to offer services for biologic fill / finish operations
The biologics fill / finish market is highly fragmented, featuring a mix of small, mid-sized, and large players. It is worth noting that this market is currently dominated by mid-sized companies (having 51-500 employees), which represent more than 40% of the industry stakeholders, worldwide.
Several partnerships have been established in the biologics fill / finish services domain, since 2013
Nearly 40% of the deals were inked in 2020. Majority of the instances captured in the report were service agreements (60%). In addition, more than 50 deals have been inked by players to offer biologics fill / finish services for vaccines.
Over 170 expansions have been reported in biologics fill / finish services domain, since 2013
More than 50% of the total expansions were focused on enhancing the dedicated capacities, thereby enabling the industry stakeholders to accommodate their growing business and address the surge in the demand for fill / finish services. Further, more than 45% of the expansions involved the establishment of new plant / facilities or adding area to the existing facilities, across different geographical locations.
The currently available biologics fill / finish capacity is estimated to be over 185 Kiloliters
Around 90% of the installed fill / finish capacity belongs to the companies with commercial scale production capabilities. In fact, close to 85% of the available capacity belongs to the large companies (having more than 500 employees).
The demand for biologics fill / finish services is expected to grow at an annualized rate of 14%
Currently, more than 60% of the overall demand for biologics fill/ finish services is generated from filling of vials. Moreover, close to 40% of the demand is likely to be generated in the Europe region.
Europe and Asia-Pacific are expected to capture more than 70% of the market share by 2035
In terms of type of biologics, antibodies and vaccines are expected to occupy a larger share (~70%) of the total biologics fill / finish services market in 2035. Further, over 25% of the biologics fill / finish services market share for therapeutic areas is captured by oncological disorders.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/256/request-sample.html
Key Questions Answered
What is fill / finish?
Who are the key players offering biologics fill / finish services?
Where are biologics fill / finish facilities located?
What is the market share of ampoules, cartridges, vials, and syringes in fill / finish?
What types of expansion initiatives are being undertaken by players in this domain?
What is the current demand for biologics fill / finish services?
What is the current and future market size for biologics fill / finish services?
The financial opportunity within the biologics fill / finish services market has been analyzed across the following segments:
Type of Packaging Container
Ampoules
Cartridges
Syringes
Vials
Type of Biologic
Antibodies
Cell Therapies
Gene Therapies
Oligonucleotides
Proteins / Peptides
Vaccines
Others
Therapeutic Area
Oncological Disorders
Autoimmune Disorders
Infectious Diseases
Cardiovascular Disorders
Other Disorders
Scale of Operation
Preclinical / Clinical
Commercial
Company Size
Large
Mid-sized
Small
Key Geographical Regions
North America
Europe
Asia-Pacific
Middle East and North Africa
Latin America
The report features inputs from eminent industry stakeholders, according to whom, a significant increase in the demand for cell and gene therapies is driving the growth of the biologics fill / finish market. The report includes detailed transcripts of discussions held with the following experts:
Gregor Kawaletz (Chief Commercial Officer, IDT Biologika)
Matt Delaney (Vice President Business Development & Marketing, Cytovance Biologics)
Purushottam Singnurkar (Research Director and Head of Formulation Development, Syngene International)
Ales Sima (Business Development Manager, Oncomed Manufacturing)
Amit Chandra (Technology Watch Manager, Yposkesi)
Jos Vergeest (International Business Developer, HALIX)
The research also includes detailed profiles of key players (listed below) engaged in offering biologics fill / finish services; each profile features a brief overview of the company, its financial information (if available), details on biologics fill / finish services, location of facilities recent developments and an informed future outlook.
AbbVie Contract Manufacturing
Asymchem
BioReliance
Boehringer Ingelheim BioXcellence
Catalent Biologics
Charles River Laboratories
Fareva
Fresenius Kabi
Glaxo SmithKline
Hetero Drugs
Intas Pharmaceuticals
Lonza
Patheon
Pierre Fabre
Recipharm
Samsun Biologics
Syngene
Takara Bio
Wacker Biotech
WuXi AppTec
WuXi Biologics
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/biologics-fill-finish-services-market/256.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com


23 января 2023
The SARM1 targeting therapeutics market is projected to grow at a CAGR of 102.1%, during the period 2033-2040, claims Roots Analysis
SARM1 inhibitors, having demonstrated the ability to prevent axonal degeneration, are being evaluated for the treatment of a number of neurodegenerative disorders. Once approved, these therapies are likely to capture a significant market share
Roots Analysis has announced the addition of “SARM1 Inhibitors Therapeutics Market, 2022 – 2040” report to its list of offerings.
Presently, several industry and non-industry stakeholders are evaluating SARM1 inhibitors as potential therapeutic agents for the treatment of neurological disorders across various preclinical studies and early stages of clinical development, worldwide. With the ongoing pace of innovation in this field, increasing R&D activity and promising pre-clinical data, several promising leads are anticipated to be commercially launched over the coming decade and SARM1 targeting therapeutics market is anticipated to witness substantial growth in the mid to long-term.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/sarm1-inhibitors-market/request-sample.html
Key Market Insights
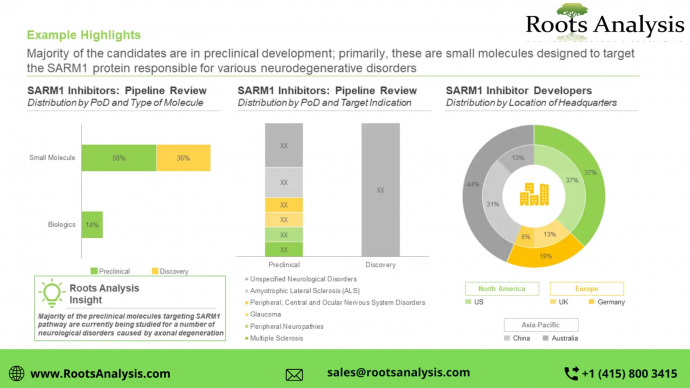
Presently, several SARM1 targeting therapy candidates are being developed by various industry players
About 65% of the pipeline candidates are currently being evaluated in the preclinical stages of development, followed by those currently in the discovery stage (35%). Further, it is worth mentioning that close to 85% of the SARM1 inhibitors are small molecules.
Currently, a number of companies claim to be engaged in the development of SARM1 inhibitors
The SARM1 inhibitors market is dominated by the presence of large companies (81%), followed by small players (19%). In addition, around 20% of the players were established post-2010.
120+ articles related to SARM1 inhibitors have been published between 2011 and 2022
Majority (81%) of the articles published in this domain were research papers, followed by review papers (12%). It is important to note that more than 70% of the total number of articles were published post-2018.
Over 30 grants have been awarded for research related to SARM1 inhibitors, since 2014
Close to 40% of the total amount was awarded under the R01 mechanism (which supports research projects). Further, genetics and neurology emerged as the key research departments, having received 34% of the total grants, each.
70+ patents related to SARM1 targeting therapeutics have been filed / granted till 2022
Over the years, the number of patents filed for SARM1 inhibitors has increased gradually; majority of the patents have been filed / granted in 2021. Most of the patents in this domain are patent applications (96%), followed by granted patents (3%).
North America is anticipated to capture more than 65% share of the market, by 2040
By 2040, the market revenues are likely to be driven by the sales of small molecules (95%) being developed as SARM1 inhibitors. Further, sales of therapeutics targeting multiple sclerosis are likely to contribute to a majority share (~40%) of revenues, in the long term.
For additional details, please visit
https://www.rootsanalysis.com/reports/sarm1-inhibitors-market.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. RNAi Market: Therapeutics and Technologies (Focus on siRNA, miRNA, shRNA and DNA) (3rd Edition): Industry Trends and Global Forecasts, 2022-2035
2. Global Therapeutic Vaccines Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com