20 января 2023
The TCR-based therapy market is projected to grow at an annualized rate of 51%, claims Roots Analysis
Given the recent approval of a TCR-based therapy, industry stakeholders have initiated several R&D efforts focused on evaluating the potential of such therapy candidates against various disease indications
Roots Analysis has announced the addition of “Global TCR-based Therapy Market (2nd Edition), 2022-2035” report to its list of offerings.
Modified T-cell receptor therapy (TCR therapy) is an emerging class of adoptive therapy that employs genetically modified lymphocytes to target specific tumor markers. Growing burden and unaddressed complex treatment needs of cancer patients have paved the way for more effective, safe and versatile alternatives, such as TCR therapies. Driven by Kimmtrak® approval, promising development pipeline and encouraging clinical trial results, the TCR-based therapy market is anticipated to witness significant growth in the mid to long-term
Key Market Insights
Over 190 TCR-based therapies are currently approved / under development
Close to 45% of the aforementioned candidates are being evaluated in clinical trials (phase I and above), while 30% of them are in preclinical stage of development. More than 90% of the therapy candidates, which are being developed to target a range of disease indications, are autologous in nature. Further, NY-ESO-1 and MAGE have emerged as the most popular target antigens.
More than 7,000 patients have been enrolled in nearly 110 clinical trials, worldwide
Clinical research activity, in terms of number of trials registered, is reported to have increased over the last five years. Of the total number of trials, close to 65% are active and still recruiting patients, while close to 26% have already been completed.
Partnership activity within this domain has grown at a CAGR of 27%, between 2015 and 2021
More than 70 agreements have been inked in the last 5 years. Majority of partnership deals signed within this domain were R&D agreements (26%), licensing agreements (21%), and product development and commercialization agreements (16%).
Over 11.2 USD billion has been invested by both private and public investors in this domain, since 2007
Specifically, in 2020 and 2021, industry players raised over USD 2.7 billion. In addition, majority (39%) of the companies primarily received funding through venture capital rounds. Further, around 70% of the total funding instances were reported by players headquartered in the US.
More than 70 patents have been filed / published related to the TCR-based therapies
Close to 85% of the filed / published patents were patent applications, while around 15% were granted patents and others. Of the total granted applications, close to 45% were filed in North America and Europe.
The market is anticipated to grow at a CAGR of 51%, during the period 2022-2035
In 2035, close to 60% of the market revenues are expected to be generated from sales of therapeutics intended for Nasopharyngeal Carcinoma and Multiple Myeloma. Further, therapies targeting for NY-ESO-1 are expected to occupy a larger share (~50%) of the overall market in 2035.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/tcr-based-therapies-market/request-sample.html
Key Questions Answered
What is the growth rate of TCR-based therapy market?
Which region has the highest growth rate in the TCR-based Therapies market?
Who are the leading industry and non-industry players in this market?
How many players are developing TCR-based Therapies?
Which target indication covers the largest TCR-based therapy market share?
What is the partnership and collaboration trend in the TCR-based therapy domain?
What is the current IP landscape of TCR-based therapies market?
The financial opportunity within the TCR-based therapies market has been analyzed across the following segments:
Target Indication(s)
Nasopharyngeal Carcinoma
Multiple Myeloma
Head and Neck Carcinoma
Sarcoma, Melanoma
Acute Myeloid Leukemia
Lung Cancer
Ovarian Cancer
Merkel Cell Cancer
Target Antigen
NY-ESO-1
EBV
gp100
Others
Key Geographical Regions
North America
Europe
Asia Pacific
Latin America
Middle East and North Africa
Rest of the World
The report features inputs from eminent industry stakeholders, according to whom, T-cell immunotherapies are expected to be the next big step in cancer immunotherapy. The report includes detailed transcripts of discussions held with the following experts:
Vincent Brichard (Vice President, Immuno-Oncology, Celyad)
Adrian Bot (Vice President, Scientific Affairs, Kite Pharma)
Victor Lietao Li (Co-Founder and Chief Executive Officer, Lion TCR)
Miguel Forte (Former Chief Operating Officer, TxCell)
The report includes brief profiles of key players (listed below); each profile features an overview of the company, details related to its financial information (if available), details about TCR-based product(s), such as information on type of therapy and current development status, information on technology portfolio (if available), recent developments related to TCR-based therapies and manufacturing capabilities of the players.
Adaptimmune Therapeutics
Alaunos Therapeutics
Bristol Myers Squibb
Cellular Biomedicine Group
Gilead Sciences
GlaxoSmithKline
Immatics
Immunocore
Lion TCR
Takara Bio
Zelluna Immunotherapy
For additional details, please visit https://www.rootsanalysis.com/reports/tcr-based-therapies-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Global T-Cell (CAR-T, TCR, and TIL) Therapy Market (6th Edition), 2022- 2035
2. mRNA Therapeutics and mRNA Vaccines Market (2nd Edition), 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com

19 января 2023
The 4D bioprinting market is anticipated to grow at a CAGR of around 35% by 2035, claims Roots Analysis
Owing to organ scarcity and lesser number of donors, the demand for tissue engineering and organ reconstruction has increased notably; as a result, the adoption of advanced 4D bioprinting technology is anticipated to increase over the coming years
Roots Analysis has announced the addition of “4D Bioprinting Market, 2023-2035” report to its list of offerings.
4D bioprinting which involves the fourth spatio-temporal frame has diverse applications, including its use in drug regeneration and dynamic bio-constructs which can alter their shape with time. It has proven to be beneficial for research and development in the biopharmaceutical and healthcare industries. Presently, several players having dedicated facilities, cutting-edge machinery, and production lines, are engaged in the development of 4D bioprinters and smart biomaterials. In addition to the ability to morph, structures from 4D printing, have the ability to self-repair and can adapt to various environmental changes.
Key Market Insights
Currently, close to 60% of the 4D bioprinters are commercially available in the market
Of these, over 45% of the 4D bioprinters are based on laser-based technology, followed by products based on polymer-based technology (20%). Further, about 60% of the 4D bioprinters use polymer as a biomaterial.
Around 40% of the players developing smart biomaterials, are headquartered in Europe
The market is currently dominated by the presence of large players (57%); of these, interestingly all the players have been established before 1980. Interestingly 68% of smart biomaterials developed in Europe are made up of polymers; of these close to 55% have already been commercialized.
Publications related to the 4D bioprinting domain have been observed to grow at a CAGR of 130%, since 2016
Around 55% of the articles focused on 4D bioprinting were research articles, of these, more than 20% of the publications have been published in 2022. Further, popular journals that have published multiple articles include Polymers, Frontiers in Bioengineering and Biotechnology, and Micromachines Gels.
Europe and Asia-Pacific are anticipated to capture a larger share (53%) of the market, by 2035
In addition, in terms of type of technology, the market is anticipated to be primarily driven by the laser-based technology capturing more than 35% of the market. Further, biomedical applications are expected to occupy a larger segment, sharing around 65% of the overall market, by 2035.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/4d-bioprinting-market/request-sample.html
Key Questions Answered
What is meant by 4D Bioprinting?
How does 4D bioprinting work?
What are the benefits of 4D Bioprinting?
Who are the leading developers of 4D bioprinters and smart biomaterials?
Which is the hub of 4D bioprinter and smart biomaterial developers?
What is the relative competitiveness of different 4D bioprinter and smart biomaterial developers?
What is the relative competitiveness of different 4D bioprinters?
What are the strengths and threats for the developers engaged in the 4D bioprinting industry?
What is the focus of various publications related to 4D bioprinting?
What is the current / future market of 4D bioprinting?
The financial opportunity within the 4D bioprinting market has been analyzed across the following segments:
Type of Technology
Extrusion-based Technology
Laser-based Technology
Inkjet-based Technology
Others
Application Area
Biomedical Applications
Others
End-user
Pharmaceutical and Biotechnology Companies
Academic Research and Development
Other end-users
Key Geographical Regions
North America
Europe
Asia-Pacific
Latin America
Middle East and North Africa
The opinions and insights presented in this report were also influenced by discussions held with eminent stakeholders in this industry. The report features detailed transcripts of interviews held with the following industry stakeholders:
Suhridh Sundaram (Chief Operating Officer, Avay Biosciences)
Preethem Srinath (Doctoral Candidate, CURAM)
Sam Onukuri (Independent Consultant)
The research also includes profiles of key players (listed below) engaged in developing 4D bioprinters and smart biomaterials; each profile features an overview of the company, financial information (if available), details on product portfolio, recent developments, and an informed future outlook.
DirectSync Surgical
Enovis
Ferentis
Poietis
REGENHU
ROKIT Healthcare
Sculpteo
SMART3D
Stratasys
VIVAX BIO
For additional details, please visit
https://www.rootsanalysis.com/reports/4d-bioprinting-market.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com


18 января 2023
The global digital twins market is projected to grow at a CAGR of 30% till 2035, claims Roots Analysis
Given the potential of digital twins to replicate the physical world in a digital layout, as well as their ability to provide real-time outputs, while gathering constant inputs from the real-world, such products have generated significant interest within the healthcare domain
Roots Analysis has announced the addition of “Digital Twins Market, 2022-2035” report to its list of offerings.
Since the first application of the digital twin concept for manufacturing in 2002, it has emerged as a crucial component for companies to achieve higher efficiency in their production processes. According to the US-based Digital Twin Consortium, digital twins hold the potential to reduce clinical trial expenditure, turnaround times, staffing expenses and plan medical intervention. Digital twin technology companies are currently engaged in the development of products which are intended for numerous applications, such as asset / process management, personalized treatment and surgical planning.
Key Market Insights
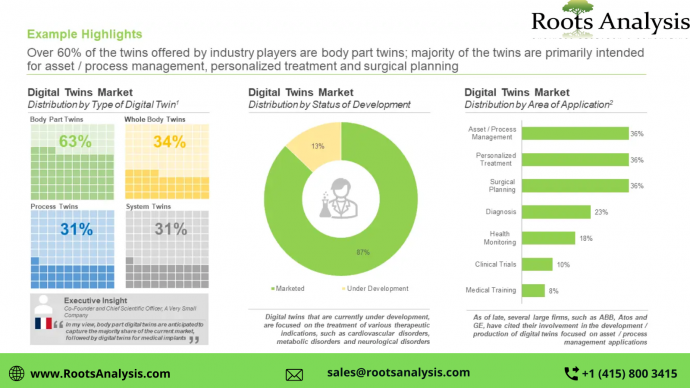
Over 35 digital twins are presently available in this market space
More than 60% of the digital twins offered by industry players are body part twins; these products are primarily intended for asset / process management, personalized treatment and surgical planning. Further, majority of the products that are currently under development are focused on the treatment of indications, such as cardiovascular disorders, metabolic disorders, and neurological disorders.
More than 30 players currently claim to be engaged in the digital twin domain
Majority of the players engaged in this industry are small firms (11-50 employees, 37%), followed by very large players (10,000+ employees, 22%), large players (501-10,000 employees, 19%), mid-sized players (51-500 employees, 13%) and very small companies (2-10 employees, 9%). Additionally, nearly 70% of the developers are based in Europe, followed by those based in North America (28%).
Partnership activity for digital twins witnessed a CAGR of over 15%, in the past three years
More than 50% of the partnerships have been signed since January 2020; of these, four partnerships were inked in the year 2022. Technology integration agreements have emerged as the most popular (30%) type of partnership model in the market, followed by technology utilization agreements (23%).
Nearly USD 6 billion invested across the globe to advance digital twins focused initiatives
A steady increase has been observed in funding activity since 2020, with the maximum number of instances (10) being reported in the year 2021. In fact, more than 90% of the total investment (in terms of the amount invested) was made in the last two years alone (2021- 2022). Of the amount raised in 2022, about USD 4.2 billion was contributed by the IPO of Babylon.
Berkus start-up valuation analysis has been performed to evaluate start-ups in this domain
The framework enables valuation of start-ups, based on several key success / risk factors . The key factors are assigned monetary values based on the qualities / risks possessed by a particular player. At the end, all valuations were added to calculate the final valuation of the company.
Players based in North America are anticipated to capture nearly 30% of the market share by 2035
The overall digital twin market is expected to be primarily driven by the growing interest towards automation and prognostic systems. The estimates in our report suggest that digital twins intended to treat cardiovascular disorders are expected to hold 32% share of the market in 2035. It is interesting to mention that market for digital twins intended to serve pharmaceutical companies and medical device manufacturers, collectively, hold over 60% of the market share in 2035.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/digital-twins-market/request-sample.html
Key Questions Answered
Who are the leading players engaged in the development of digital twins for the healthcare domain?
Which type of digital twin is most commonly offered by developers engaged in this market space?
What is the relative competitiveness of different players engaged in the digital twins domain?
What is the likely valuation of start-ups involved in the development of digital twins for the healthcare sector?
What is the present and likely future demand for digital twins in the overall healthcare sector?
What are the anticipated future trends related to digital twins in the healthcare domain?
How is the current and future opportunity likely to be distributed across key market segments?
The financial opportunity within the digital twins market in the healthcare domain has been analyzed across the following segments:
Therapeutic Area
Cardiovascular Disorders
Metabolic Disorders
Orthopedic Disorders
Other Disorders
Type of Digital Twin
Process Twins
System Twins
Whole Body Twins
Body Part Twins
Area of Application
Asset / Process Management
Personalized Treatment
Surgical Planning
Diagnosis
Other Applications
End Users
Pharmaceutical Companies
Medical Device Manufacturers
Healthcare Providers
Patients
Other End Users
Key Geographical Regions
North America
Europe
Asia
Latin America
Middle East and North Africa
Rest of the World
The research includes profiles of key players (listed below); each profile features a brief overview of the company, details related to its recent developments (including funding and collaborations) and an informed future outlook.
Babylon
ExactCure
ImmersiveTouch
Navv Systems
ThoughtWire
Unlearn.AI
For additional details, please visit https://www.rootsanalysis.com/reports/digital-twins-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Flow Cytometry Service Market, 2022-2035
2. Gene Editing beyond CRISPR Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact Information
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com

17 января 2023
THE INTRICACIES ASSOCIATED WITH CELL THERAPY MANUFACTURING HAVE PAVED THE WAY FOR NOVEL AUTOMATION TECHNOLOGIES
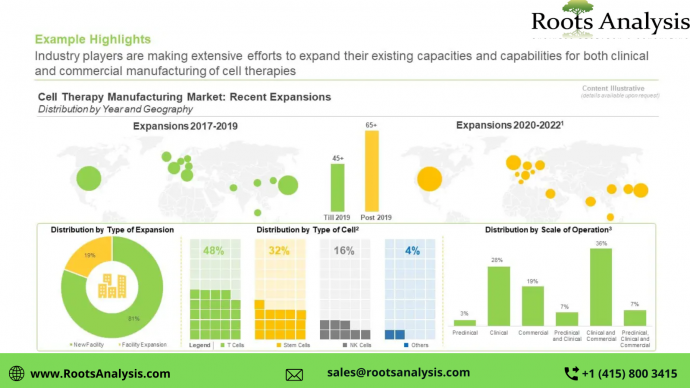
Over the years, several advanced and innovative automation tools and technologies have been developed; these have been demonstrated to hold the potential for significant reduction in the cost associated with the manufacturing of advanced therapy medicinal products, thereby, making such products more affordable.
Considering the vast potential of cellular therapies in the treatment of rare disorders and sufficient body of evidence validating the clinical benefits / therapeutic potential of this complex class of biologic drugs, cell therapies have garnered considerable attention of players engaged in the healthcare industry, in the past few years.
The focus of stakeholders has now shifted to optimizing the cell therapy manufacturing process. It is important to mention that we have forecasted the evolution of the overall cell therapy manufacturing market, focusing on T-cell immunotherapies, dendritic cell therapies, NK cell therapies and stem cell therapies. Considering the sufficient body of evidence validating the clinical benefits / therapeutic potential of this complex class of biologic drugs, the focus of stakeholders has now shifted to optimizing the cell therapy manufacturing process.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/285/request-sample.html
One such emerging concept, namely GMP-In-A Box, offers several advantages, including increased throughput, decreased idle time between batch runs and reduced manual labor. However, the delicate nature of steps involved in the cell therapy production process is known to hinder the overall automation process. Further, the lack of specialized infrastructure and limited expertise available in this domain are some of the known challenges impacting the growth of this segment.
Despite the challenges associated with the development and production of such therapies, we believe, that the benefits offered by them outweigh the hurdles. They are likely to serve as important drivers of the industry’s growth. Efforts to introduce automation technologies in cell therapy manufacturing are underway, and if implemented successfully, can significantly help in the elimination of human intervention and reduce the risk of contamination. As a consequence, it is likely to result in a marked increase in product consistency, ensure the maintenance of sterility, and decrease the production time and cost. In a nutshell, cell therapies are expected to soon represent one of the prominent therapeutic options within the mainstream healthcare.
For additional details, please visit https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
2. AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com

16 января 2023
The liposome development and manufacturing services market is anticipated to grow at a CAGR of nearly 10% till 2035, claims Roots Analysis
Driven by the development of advanced methods for preparation and characterization of liposomes, and the application of these molecules in therapeutics and diagnostics, the demand for liposomes is expected to rise in the coming years.
London
Roots Analysis has announced the addition of “Liposome Manufacturing Market, 2022-2035” report to its list of offerings.
Despite the growing interest in liposome-based therapeutics and diagnostics, the development and manufacturing of liposomes is associated with several challenges, including complex manufacturing processes, huge capital investments, inadequate clinical grade production and GMP compliant industrial scale-up, lack of facilities with necessary infrastructure, as well as concerns related to storage and stability. In order to deal with the aforementioned challenges, a number of pharmaceutical companies have demonstrated the preference to outsource their respective liposome development and manufacturing operations to specialized service providers.
Key Market Insights
More than 70 companies are currently offering services related to liposome development and manufacturing, globally
Majority (74%) of the service providers offer formulation development services for liposomes. This is followed by players offering analytical method development (65%), liposome contract manufacturing (64%), and process development (61%) services.
Close to 6,100 articles focused on liposomes, have been published in reputed scientific journals, since 2017
More than 50% of the articles focused on liposomes were published post-2019. Popular journals that have published multiple articles include International Journal of Pharmaceutics, Journal of Controlled Release, and Pharmaceutics.
More than 800 clinical trials have been registered for the evaluation of liposome-based therapeutics, worldwide
The clinical research activity, in terms of number of trials registered, is reported to have increased at a CAGR of 17%, during the period 2017-2021. Of the total number of trials registered, 60% have already been completed, while 26% of the studies are actively recruiting participants.
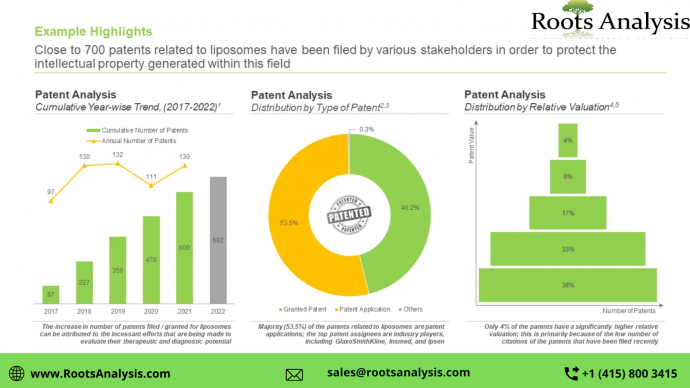
Nearly 700 patents related to liposomes have been filed / granted, since 2017
Owing to the increase in research and development efforts led by several industry and non-industry players engaged in this domain, close to 55% of patent applications have been filed post-2016. It is worth noting that 61% patents related to liposomes were filed / granted in the US alone.
Over 110 global events related to liposomes were organized in the past couple of years
Majority (63%) of the events related to liposomes, were organized virtually in order to comply with the guidelines in the ongoing COVID-19 pandemic. Further, the agendas of the events organized post-2020 include discussions on the applications, and recent advancements in technologies associated with liposomes.
Go / No-Go framework can be used to assist players in the crucial outsourcing decision-making process
The framework enables evaluation of the current capabilities of liposome-based therapeutic developers, based on 4+ parameters, including trial phase, target therapeutic area, location of trial(s), patient enrollment and willingness to outsource.
North America is anticipated to capture over 40% of the global market share for liposome development and manufacturing services, by 2035
In terms of type of product formulation, the therapeutic formulations of liposomes (71%) are anticipated to capture the highest share, this trend is unlikely to change in the foreseen future. Further, based on end users, majority of the revenue share (76%) of the overall market is likely to be driven by pharmaceutical and biotechnology industry, by 2035.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/liposome-manufacturing-market/request-sample.html
Key Questions Answered
Who are the key players offering liposome development and manufacturing services?
What is the evolving trend of publications focused on liposomes?
How many patents, related to liposomes, have been filed / granted in the past few years?
Which companies are actively involved in conducting clinical trials for liposomes?
What are the key agenda items being discussed in various global events / conferences related to liposomes?
Which factors are likely to influence the decision of liposome manufacturing being done in-house or outsourced?
How is the current and future market opportunity related to liposome development and manufacturing services, likely to be distributed across key market segments?
The financial opportunity within the liposome development and manufacturing services market has been analyzed across the following segments:
Type of Product Formulation
Therapeutic
Nutraceutical
Scale of Operation
Discovery / Research
Preclinical
Clinical
Commercial
End User
Pharmaceutical and Biotechnology Industry
Cosmetic Industry
Food Industry
Agricultural Industry
Academics
Other Industries
Key Geographical Regions
North America
Europe
Asia-Pacific
Middle East and North Africa
Latin America
The research also includes detailed profiles of the key players (listed below) offering services for liposome development and manufacturing; each profile features an overview of the company, its financial information (if available), details on service portfolio, recent developments and an informed future outlook.
Baxter BioPharma Solutions
Charles River Laboratories
Evonik
Fresenius Kabi
Fujifilm
GEA
Intertek
For additional details, please visit
https://www.rootsanalysis.com/reports/liposome-manufacturing-market.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Biologics Fill Finish Services Market (3rd Edition), 2022-2035
2. Bioavailability Enhancement Technologies and Services Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com